Session Information
Date: Monday, November 13, 2023
Title: (1221–1255) Pediatric Rheumatology – Clinical Poster II: Connective Tissue Disease
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Rituximab has been associated with high rates of infection among adults with systemic lupus erythematosus. However, studies of infection risk following rituximab among children with pediatric lupus (pSLE) are lacking. We conducted a retrospective study to assess incidence of hospitalized serious infections following rituximab among children with pSLE, and to assess changes in rituximab use over time.
Methods: Children and adolescents ages 2-21 years with an ICD-9 or ICD-10 code for SLE (710.0, M32*) and who received at least one dose of rituximab during admission to a Pediatric Health Information System (PHIS)-participating hospital from 2009-2020 were included. The PHIS includes in-hospital administrative data from >50 freestanding U.S. children’s hospitals. Serious infections were defined by ICD-9 and ICD-10 codes for infections during an inpatient, observation or admission encounter. Antimicrobial medication use was also required for bacterial or fungal infections. The study period for infections was 12 months following the index hospitalization when rituximab was administered. Summary statistics were used to describe children with pSLE who received rituximab, and the subset who developed a serious infection. Incidence rates for infections requiring hospitalization over the 12 months following first rituximab administration were calculated using exposure time truncated at time of death, first hospitalized infection, or 12 months after index hospitalization for rituximab administration. Rituximab use by year of hospital discharge was assessed from 2009-2021.
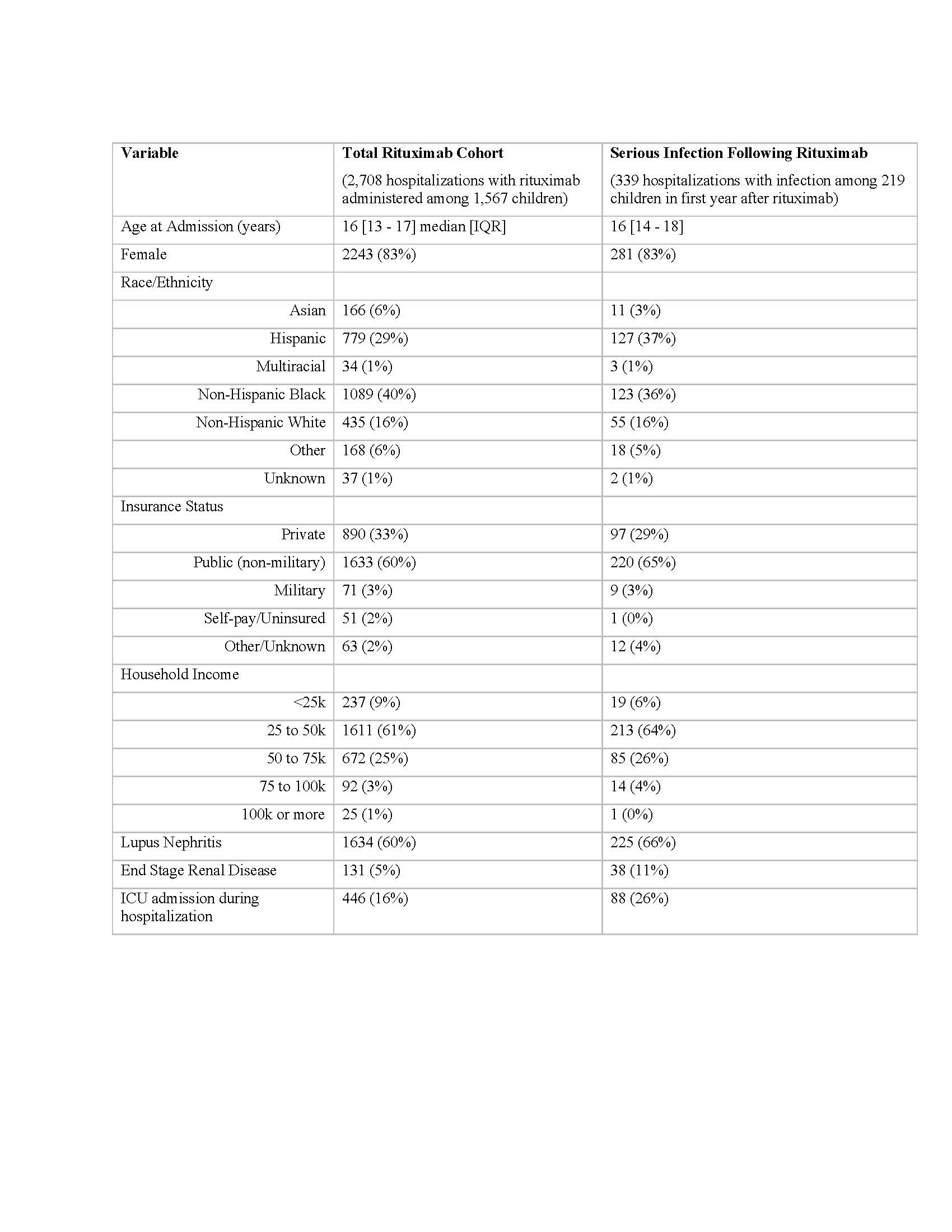
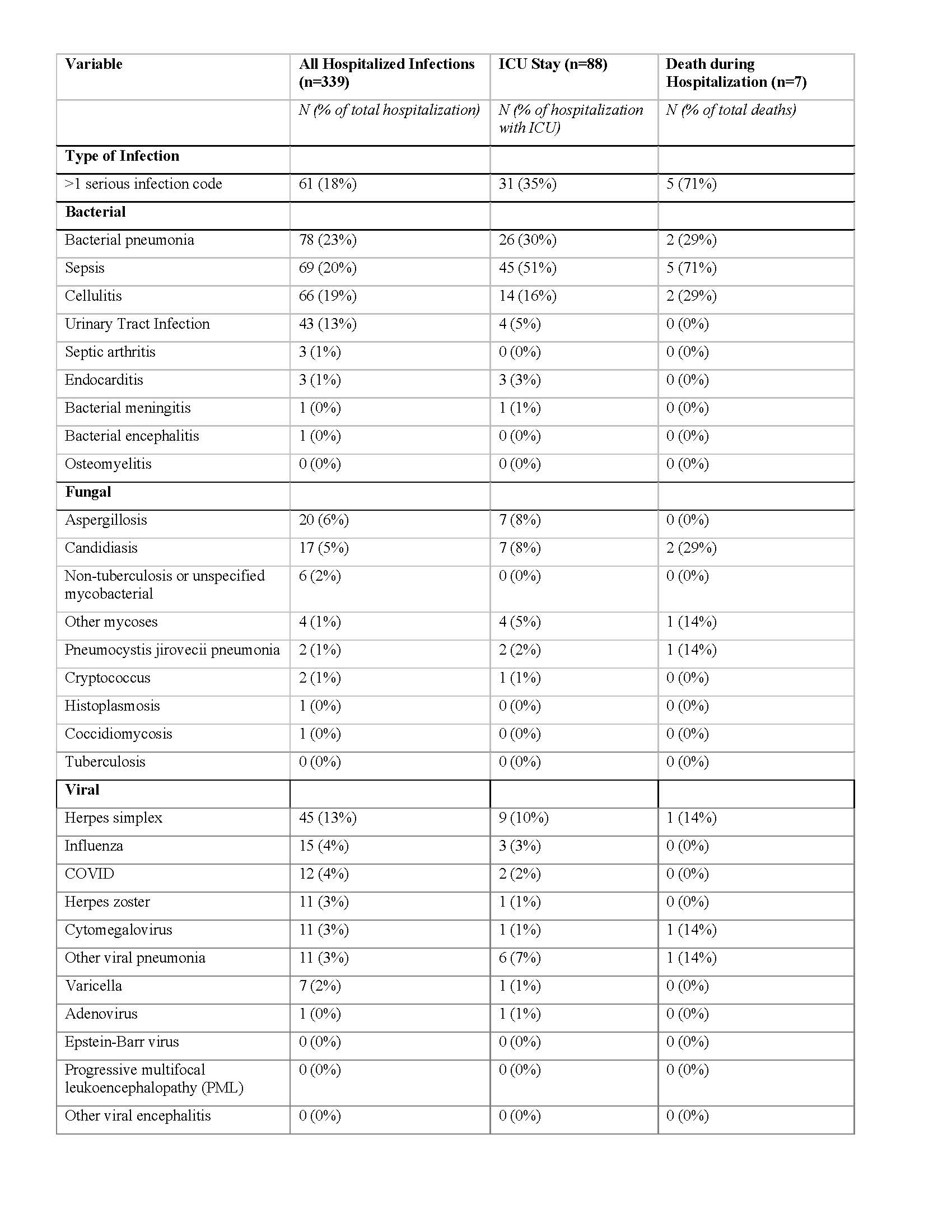
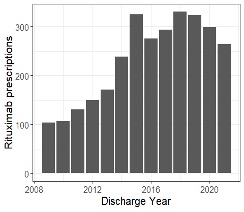
Results: We identified 1,567 children with pSLE who received rituximab. Demographic and clinical characteristics of the study cohort are presented in Table 1. 624 (40%) children with pSLE received cyclophosphamide in the 3 months prior to, and up to 1 year after index hospitalization. Hospitalizations with rituximab administered decreased from 2019 to 2020 and 2021 (Figure 1). Among 1,567 rituximab-treated children with pSLE, 219 (representing 339 hospitalizations) were admitted with a serious infection within 1 year after the first rituximab administration (exclusive of index hospitalization) for an incidence rate of 140 cases per 1,000 patient-years. Median [IQR] time to hospitalization with serious infection following rituximab was 1.83 [0.61, 5.84] months. 7 children with pSLE (0.44%) died during a hospitalization with an infection in the year following rituximab administration. The most common serious infections were bacterial pneumonia, sepsis and cellulitis (Table 2). There were 12 children with pSLE hospitalized with COVID-19 (4% of hospitalizations) and no in-hospital deaths with COVID-19.
Conclusion: We observed high rates of serious infection in the year following first rituximab administration in a large multicenter cohort of youth with pSLE. Rituximab use declined during the COVID-19 pandemic, though no fatalities during hospitalizations with COVID-19 were observed. Concomitant use of other highly immunosuppressive medications was common in our cohort. Further research is needed to identify risk factors for serious infection following rituximab among children with pSLE.
To cite this abstract in AMA style:
Roberts J, Faino A, Bryan M, Cogen J, Morgan E. Serious Infections Following Rituximab Administration in Children with Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/serious-infections-following-rituximab-administration-in-children-with-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serious-infections-following-rituximab-administration-in-children-with-systemic-lupus-erythematosus/