Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Complement plays a central role in SLE, generating an array of bioactive soluble and cell-bound complement activation products (CB-CAPs) during disease activity. Data are lacking though detailing the types, quantities, and impacts of the numerous CB-CAP on SLE immune cells, especially with respect to disease activity. We applied a mass cytometry (MC) panel that can detect over 20 CB-CAPs and complement receptors to PBMCs from paired flare and remission samples from 6 patients with classified SLE. Furthermore, we analyzed single-cell transcriptional profiles on flaring samples using antibodies to the most prevalent CB-CAPs using cellular indexing of transcriptomes and epitopes (CITE)-seq.
Methods: Adults with ACR- or SLICC-classified SLE (n=6) were consented for PBMC collection at Washington University School of Medicine. Isolated PBMCs were subjected to single cell MC and CITE-seq (n=3). MC data analysis was performed with Cytobank. CITE-seq data analyses was performed with Seuret, gProfileR, and Comprehensive Multi-omics Platform for Biological InterpretatiOn (COMPBIO).
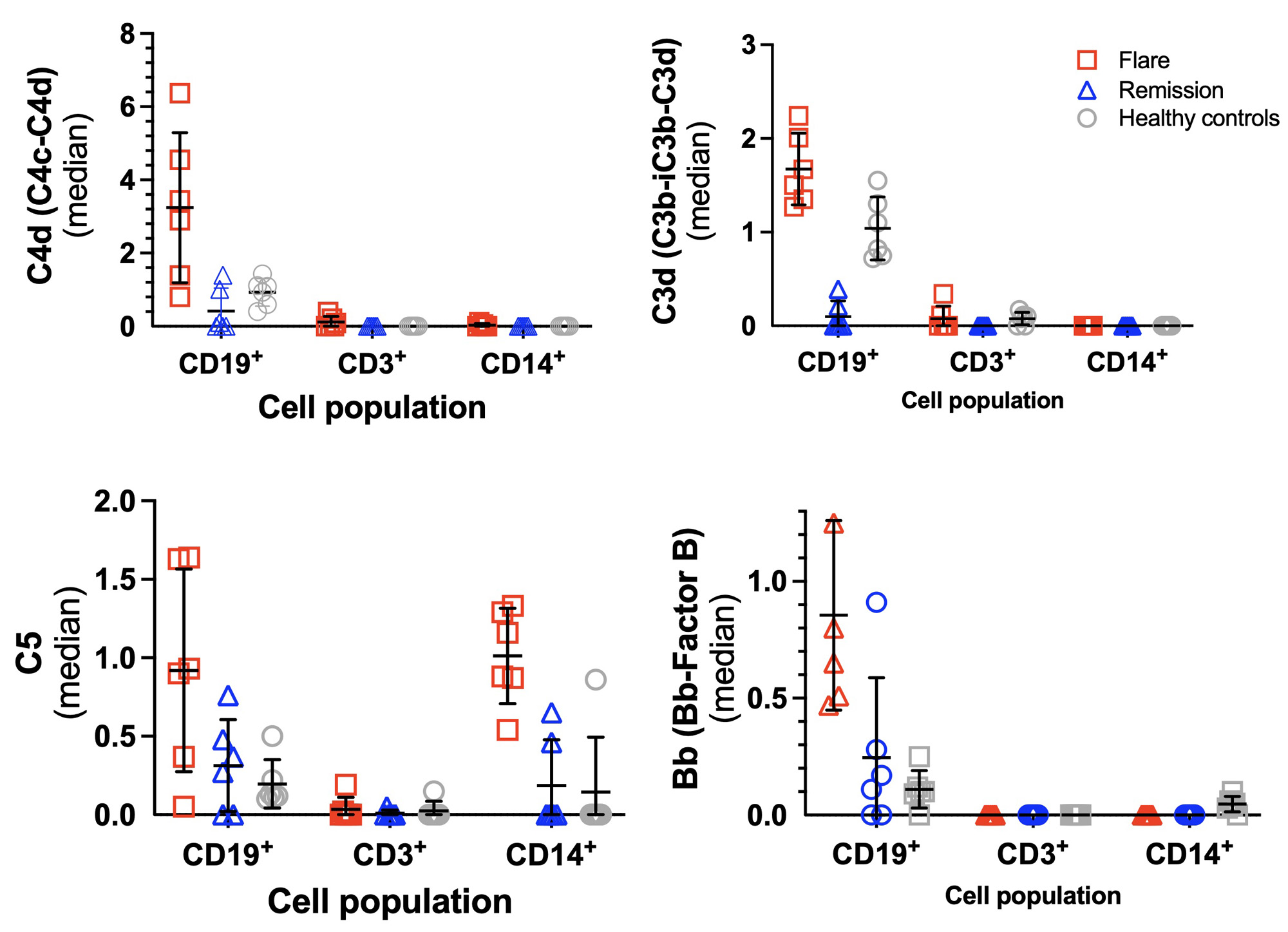
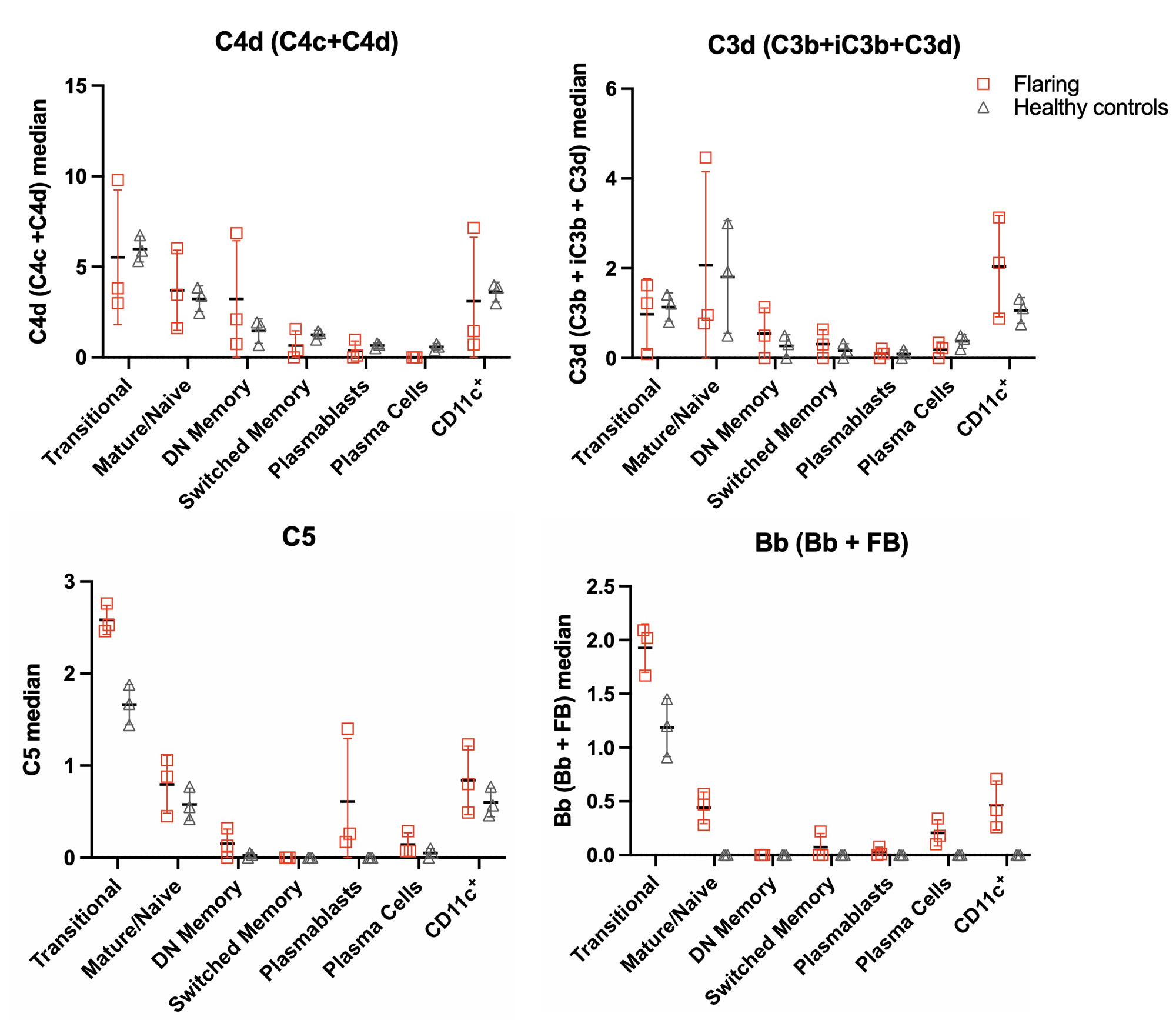
Results: We found the highest frequency of C4d, C3d, C5, and Bb deposition on B cells compared to T cells and monocytes during SLE flares (Fig 1). During disease remission, low levels of all CB-CAPs were observed in these cells. Compared to controls, transitional B cells from flaring patients with SLE had high levels of C5 and Bb with little C4d or C3d (Fig 2). CD11c+ B cells from flaring patients also had elevated Bb deposition compared to controls. CITE-seq transcriptional profiling identified Bb- and C3d-bearing CD11c+ B cells possessing a type I interferon signature, with Bb-bearing B cells further possessing a TNF/NF-κB transcriptional signature.
Conclusion: A high level of CB-CAP deposition was observed in B cells obtained from flaring subjects with a SLE, which was absent during disease remission. The types of CB-CAPs found on PBMCs were not uniform between cell types, potentially opening a previously undescribed heterogeneity in SLE. Additional heterogeneity was observed in the transcriptional profiles associated with specific CB-CAPs on B cells. These pilot data demonstrate the feasibility of the MC complement panel on human samples, and the potential insights CITE-seq has using CB-CAPs in discovering novel mechanisms of complement activation and regulation.
To cite this abstract in AMA style:
Arguelles G, Mitchell L, Hourcade D, Atkinson J, Roberson E, Kim A. Distinct Cell-Bound Complement Activation Products Associate with Disease Activity and Immune Transcriptional Signatures in SLE [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/distinct-cell-bound-complement-activation-products-associate-with-disease-activity-and-immune-transcriptional-signatures-in-sle/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/distinct-cell-bound-complement-activation-products-associate-with-disease-activity-and-immune-transcriptional-signatures-in-sle/