Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Atherosclerosis and vascular inflammation are a cause of morbidity and mortality in systemic lupus erythematosus (SLE). Vascular NETosis is a driver of atherosclerosis and vascular inflammation. Isolevuglandins (IsoLGs) are lipid-aldehydes formed by peroxidation of arachidonic acid or prostaglandin-H2. IsoLGs are efficiently scavenged by 2-hydroxybenzylamine (2-HOBA). Treatment of SLE-prone B6.SLE123 mice with 2-HOBA attenuates systemic inflammation and reduces blood pressure. Additional studies have shown that isoLGs directly contribute to the formation of neutrophil extracellular traps (NETosis). We hypothesized that isoLGs contribute to neutrophil expansion and aortic NETosis in SLE-prone mice.
Methods: We treated 8-week-old female SLE prone B6.SLE123 mice with 1g/L 2-HOBA in the drinking water for 4-weeks. C57BL/6 mice were used as healthy controls. Spleens were harvested and splenocytes were submitted for single cell sequencing. Data were analyzed with Seurat v4.0.0 in R v4.0.4. NETosis in mouse tissue was measured by flow cytometry. Peripheral blood was obtained from a research subject with SLE that was recruited as part of the Memantine for the Treatment of Cognitive Impairment in Systemic Lupus Erythematosus clinical trial (IRB# 180256). To determine the role of isoLGs in NETosis, neutrophils were harvested from peripheral blood. Cells were seeded onto poly-L-lysine coated plates and incubated at 37°C for 2-hours in the presence of 200 μM ethyl-2-hydroxybenzylamine (ethy-2-HOBA), an isoLG scavenger with improved nuclear penetrance, or phosphate buffered saline as a control. Human NET formation was determined with immunofluorescence and quantified using the Imaris Cell Imaging Software (Oxford Instruments).
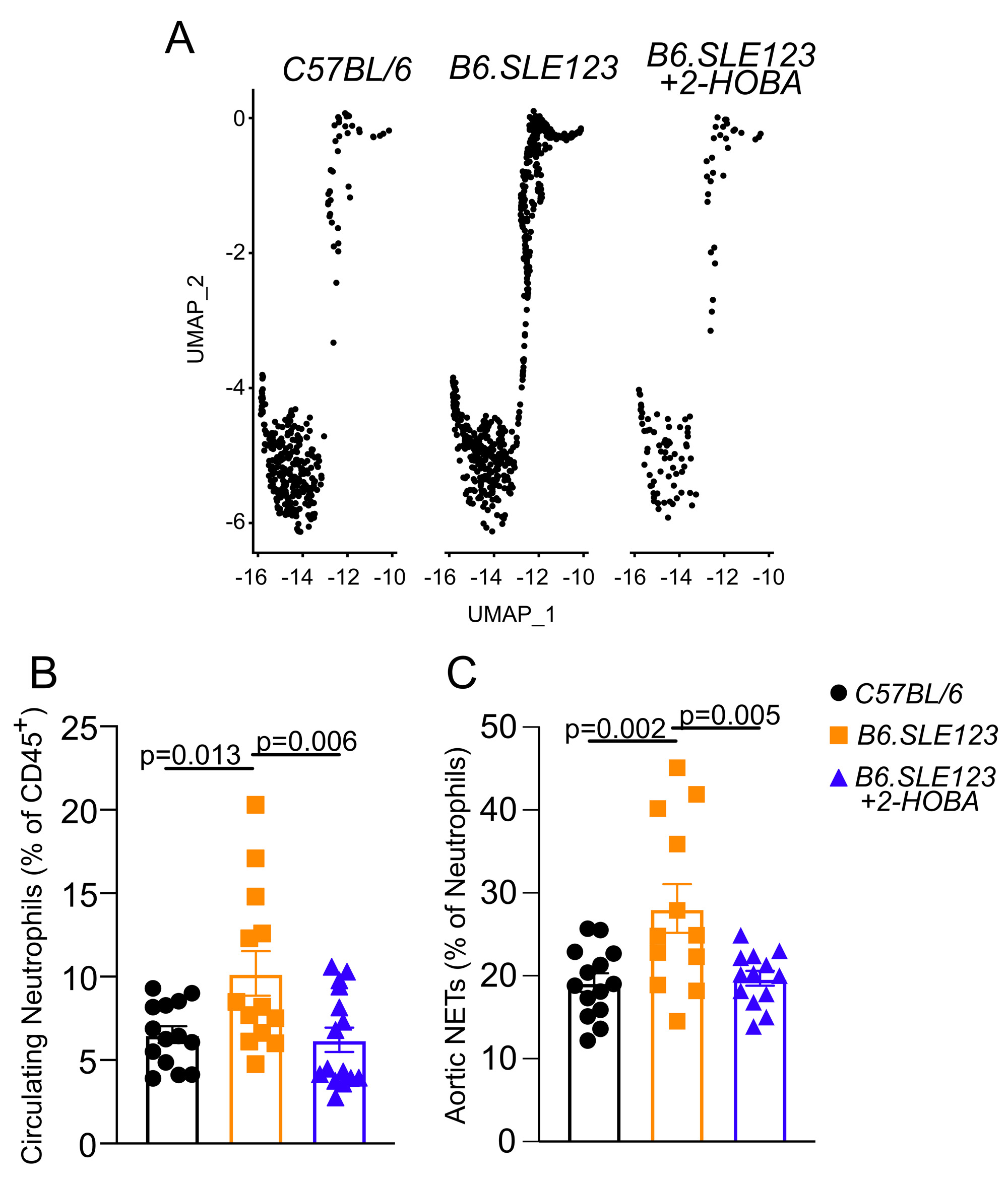
Results: Single cell sequencing revealed an increase in splenic neutrophils in B6.SLE123 mice compared to C57BL/6 controls. Treatment with 2HOBA attenuated this increase (C57BL/6 2.1%, B6.SLE123 3.8%, B6.SLE123+2HOBA 0.6% of total sequenced cells, N=3) (Figure 1A). Flow cytometry revealed an increase in circulating neutrophils in B6.SLE123 mice that was attenuated with 2HOBA (C57BL/6 6.5 ± 1.9%, B6.SLE123 10.2 ± 4.8%, B6.SLE123+2HOBA 6.2 ± 2.8% of CD45+ cells, N = 12-14)(Figure 1B). Aortic NETs were also increased in SLE prone mice which was attenuated following 2HOBA treatment (C57BL/6 19.2 ± 4.2%, B6.SLE123 28.1 ± 10.17%, B6.SLE123+2HOBA 19.7 ± 3.2% of neutrophils, N = 12-14)(Figure 1C). Human SLE subject neutrophils showed a reduction in total NET area after treatment with ethyl-2-HOBA (Control 4,415 ± 1238 µm2/field vs 2,215 ± 1372 µm2/field, n = 5, p = 0.029).
Conclusion: In a murine model of SLE, scavenging of isoLGs reduces neutrophils in secondary lymphoid tissue and peripheral blood. Additionally, we observe a reduction in vascular NETosis. Treatment of human neutrophils with an isoLG scavenger attenuates NET formation. These findings describe a role of isoLGs in SLE-associated NETosis and resultant vascular inflammation. Moreover, these findings suggest a potential role for isoLGs in NET-driven autoinflammation and vascular disease in SLE.
To cite this abstract in AMA style:
Krishnan J, de la Visitación N, Williams J, Crofford L, Patrick D. Scavenging of Isolevuglandins Attenuates Neutrophil Expansion and Aortic NETosis in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/scavenging-of-isolevuglandins-attenuates-neutrophil-expansion-and-aortic-netosis-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/scavenging-of-isolevuglandins-attenuates-neutrophil-expansion-and-aortic-netosis-in-systemic-lupus-erythematosus/