Session Information
Date: Sunday, November 12, 2023
Title: (0252–0282) Miscellaneous Rheumatic & Inflammatory Diseases Poster I
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with rheumatic diseases (RD) on rituximab (RTX) have increased mortality following COVID-19 and reduced antibody response post-vaccine. We tested whether a mix-and-match strategy using a novel protein subunit vaccine (PSV) is safe and enhances vaccine-specific responses in those who have received >3 messenger RNA (mRNA) vaccine doses.
Methods: We recruited adults with RDs on RTX post-3rd/4th dose of a mRNA COVID-19 vaccine in an open label, non-randomized, comparative trial. Post-3rd dose participants chose to receive either a 4th dose of mRNA Spikevax® (Moderna mRNA-1273) or PSV Nuvaxovid® (Novavax NVX-CoV2373) vaccines. Patients enrolled post-4th mRNA dose were offered Nuvaxovid®as their 5th. Self-reported reactogenicity at 7 days and adverse events at 28 days were noted. Humoral and cellular vaccine-responses were determined pre and 28 days post-vaccine. We tested for anti-spike (anti-S) by plasma binding on D614G Spike transfected HEK293T cells and measured by flow cytometry, anti-receptor binding domain (anti-RBD) and anti-nucleocapsid (anti-N) by in-house ELISA, and serum neutralizing antibodies (NAbs) using Wuhan and BA.5 pseudotyped lentiviruses. In 19 participants receiving a 4th dose, we tested for spike-specific B, CD4 and CD8 T cell responses by flow cytometry using RBD-B and activation-induced marker (AIM) assays. We used descriptive statistics for demographics, reactogenicity and immunogenicity and ANOVA tests to assess the impact of a booster dose on vaccine-induced immune responses.
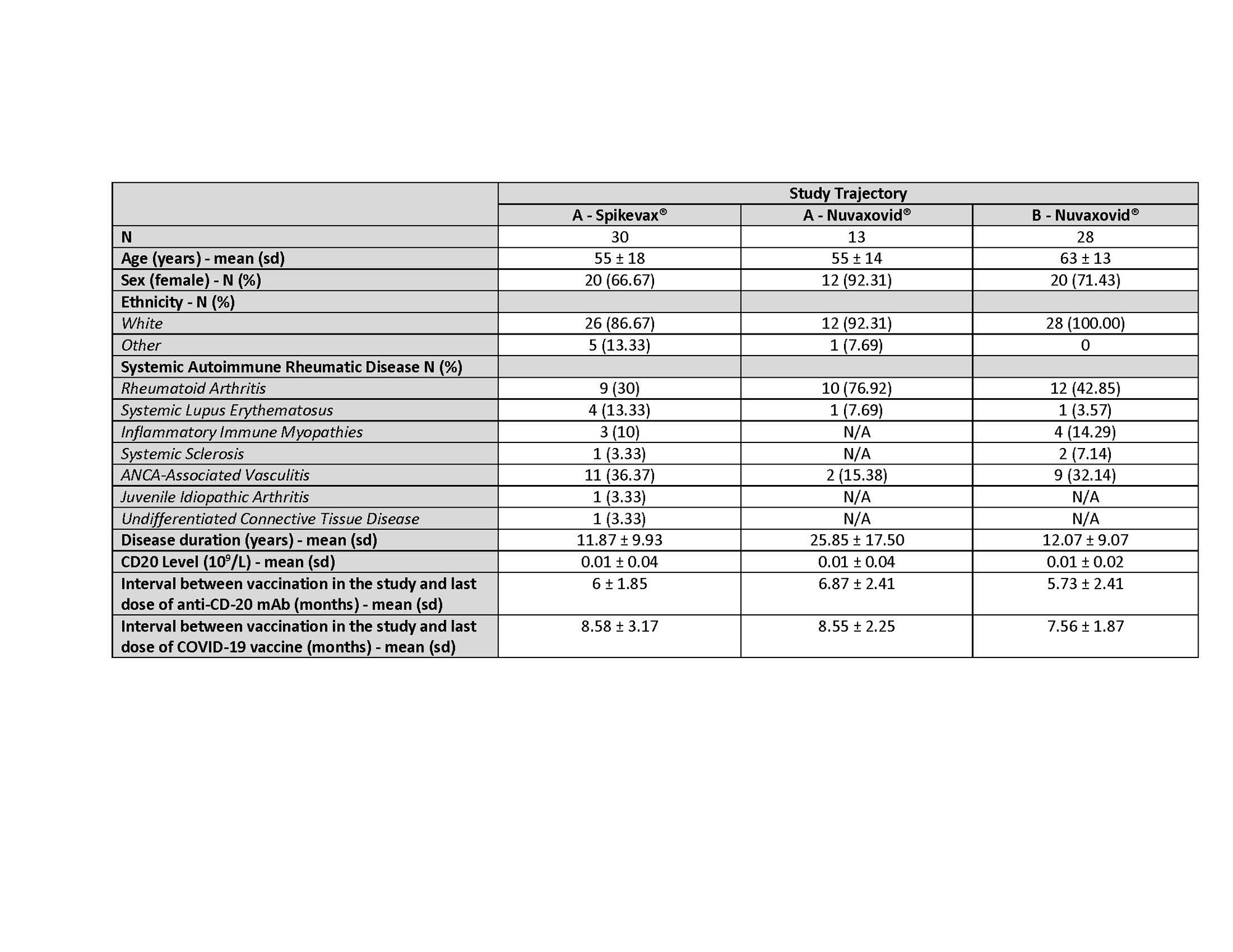
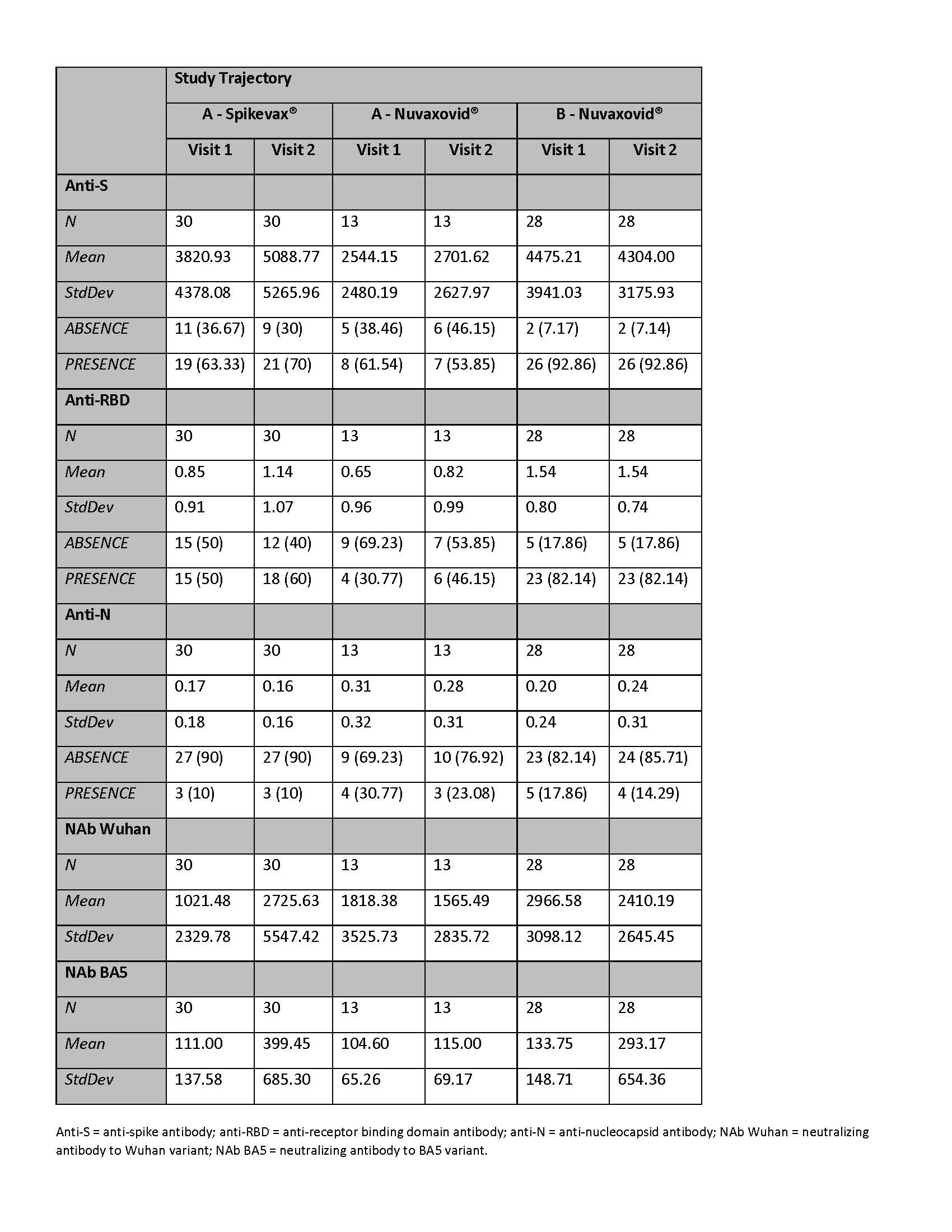
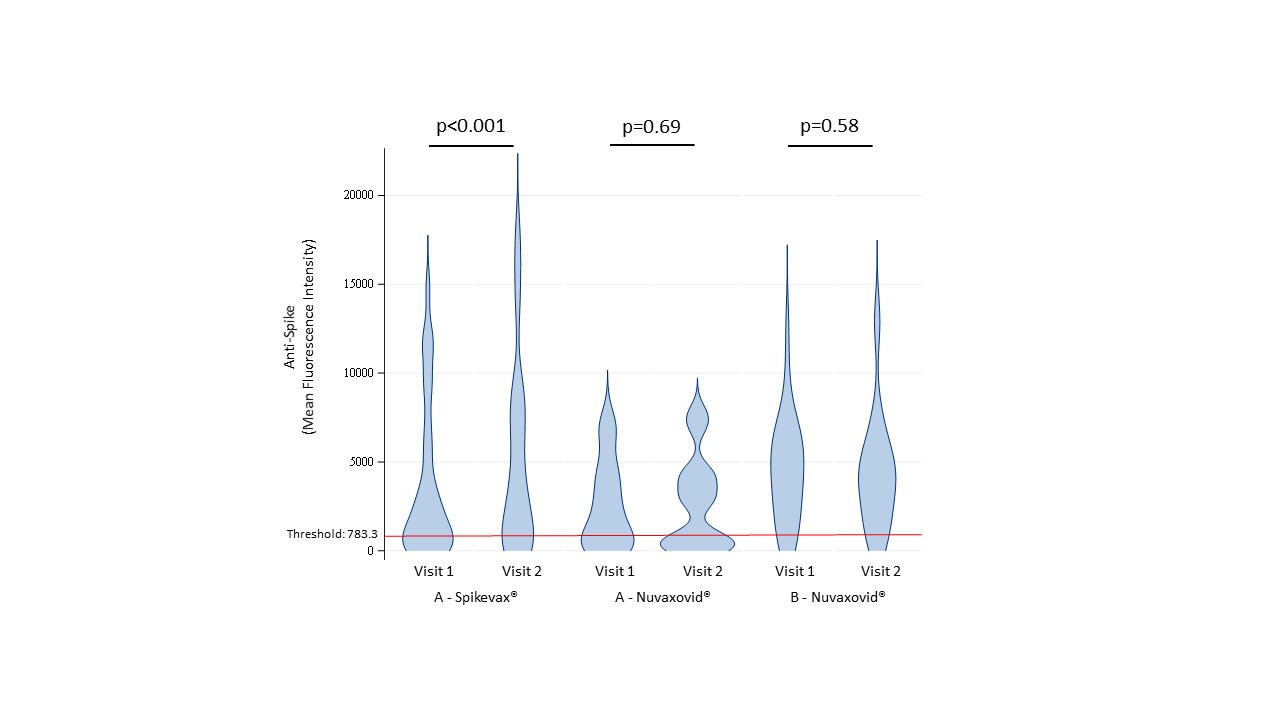
Results: Table 1 summarizes data on the first 71 participants (4th dose Spikevax® = 30, 4th dose Nuvaxovid® = 13 and 5th dose Nuvaxovid® = 28). Reactions to the vaccine at 7 days were frequent but not severe. Most common was fatigue, headache, injection site pain and arthralgias. At day 28, five new COVID-19 infections (one requiring hospitalization) and seven disease flares were reported. Table 2 reports anti-S, anti-RBD, anti-N, and NAbs to Wuhan and BA.5. Anti-S levels (Figure 1) were higher in those receiving 5th versus 4th dose. In those to whom we gave a 4th vaccine, we observed higher anti-S levels post mRNA (p < 0.001) but this was not clearly seen when the 4th dose was PSV. Most (16/19) of those tested post 4th dose had no detectable RBD B cell responses while three patients had higher B cells numbers and measurable RBD B cell responses. In contrast, the cohort demonstrated good Spike-specific intact and measurable AIM+ CD4 and CD8 T cell responses that did not increase post 4th dose.
Conclusion: Mix-and-match booster dosing was not clearly associated with unusual vaccine reactions, adverse events or disease flares in RTX-treated RD patients. In those who had already received 3 mRNA doses, there was a significant increase in anti-S levels after receiving a 4th mRNA vaccine but not when the 4th vaccine was PSV. Humoral and RBD-B-cell responses increased with a fourth dose. AIM+ CD4 and CD8 T cell responses were intact, showing a disconnect with weak B cell frequencies. We did not see a clear benefit for PSV compared to mRNA booster (after primary mRNA vaccine series) in RTX-treated RD patients. This may have important implications for COVID vaccine strategies in 2023-2024.
To cite this abstract in AMA style:
Amiable N, Benlarbi M, Dube M, Theriault S, Godbout A, Julien A, Ortega-Delgado G, Duchesne M, Cloutier R, Perreault J, Gravel A, Fournier L, Alfonso G, Bourre-Tessier J, Hudson M, Richard N, Makhzoum J, Mendel A, Bernatsky S, Dionne M, Libman M, De Serres G, Dieudé M, Flamand l, Kaufmann D, Finzi A, Bazin R, Colmegna I, Fortin P. A Mix-and-match COVID-19 Vaccination Strategy in Patients with Rheumatic Diseases on Rituximab [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/a-mix-and-match-covid-19-vaccination-strategy-in-patients-with-rheumatic-diseases-on-rituximab/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-mix-and-match-covid-19-vaccination-strategy-in-patients-with-rheumatic-diseases-on-rituximab/