Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The pathophysiology of viral infection is related to elevated levels of inflammatory cytokines therefore also of the excessive activation of monocytes. Prolonged inflammation can cause fibrosis, a process involving the accumulation of fibrous connective tissue and excess of extracellular matrix (ECM). The effector cells of fibrosis are myofibroblasts, an activated phenotype of fibroblasts. Monocytes can be modulated in their action by the release of paracrine factors from adult stem cells. Human dental pulp stem cells (hDPSCs) appear to be cells of low immunogenicity and easily isolated. The aim of this study was to observe the action exerted by monocytes in an inflammatory and profibrotic environment of viral induction and the effect on fibroblasts, as well as the modulating role of hDPSCs on monocytes and consequently on fibrosis.
Methods: An in vitro model of fibroinflammation was set up using human fibroblasts and monocytes isolated respectively from the skin and blood of healthy donors. Monocytes were activated with an imidazoquinoline (CL097) to simulate a viral infection and then characterized by cytofluorimetric analysis. Fibroblasts were treated with activated monocyte conditioned medium and the effect was verified by real-time PCR and western blot. Monocytes and hDPSCs were then co-cultured for 48 hours. Subsequently, the fibroblasts were treated for different times with the conditioned medium obtained from the co-cultures. The expression level of the main inflammatory mediators and profibrotic factors was determined by real-time PCR, western blot, immunofluorescence and ELISA immunoassay.
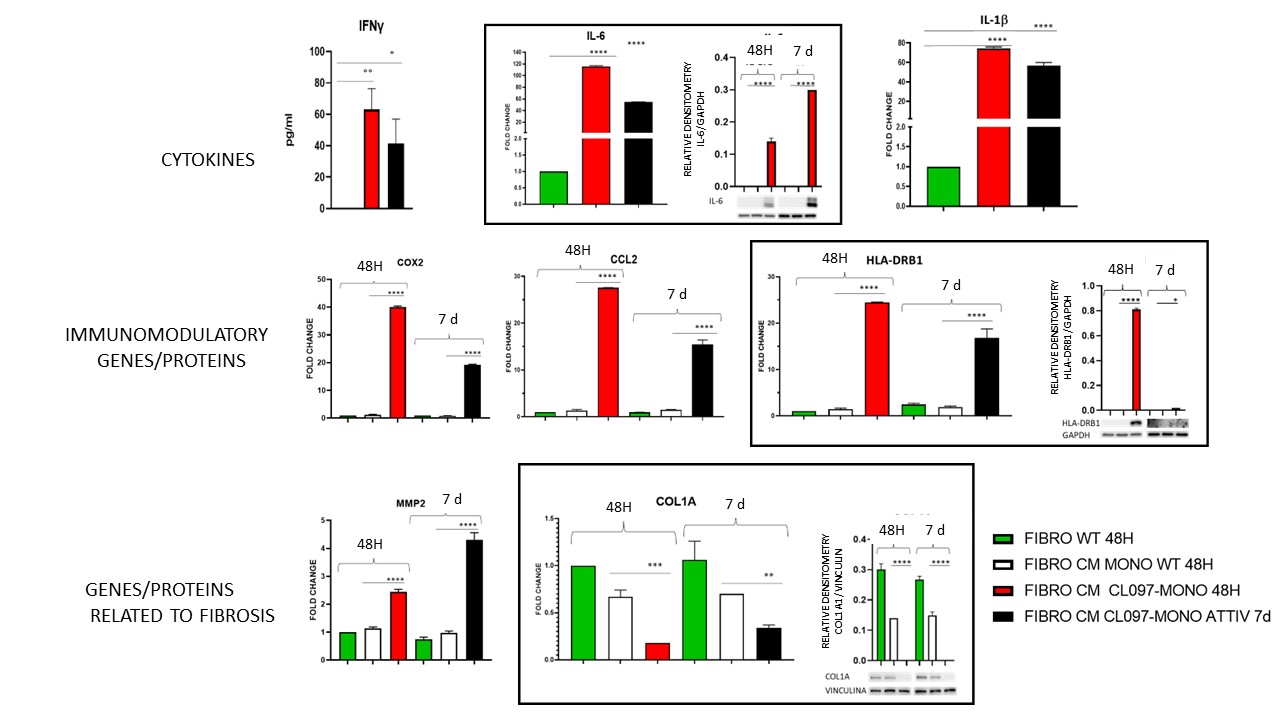
Results: Already after 48 hours of treatment with the conditioned medium of activated monocytes, a significant increase in the expression of proinflammatory cytokines (IFNγ, IL-6 and IL-1β), immunomodulatory genes (CD55, COX2, CCL2 and HLA-DRB1), Col1A1 and metalloproteinase 2 (MMP2) was observed in fibroblasts, while only prolonged exposure to the conditioned medium for seven days induced an increase in the other profibrotic genes FN1, ICAM1 and αSMA (IFNγ p < 0,05, the others p < 0,0001) (FIG.1).
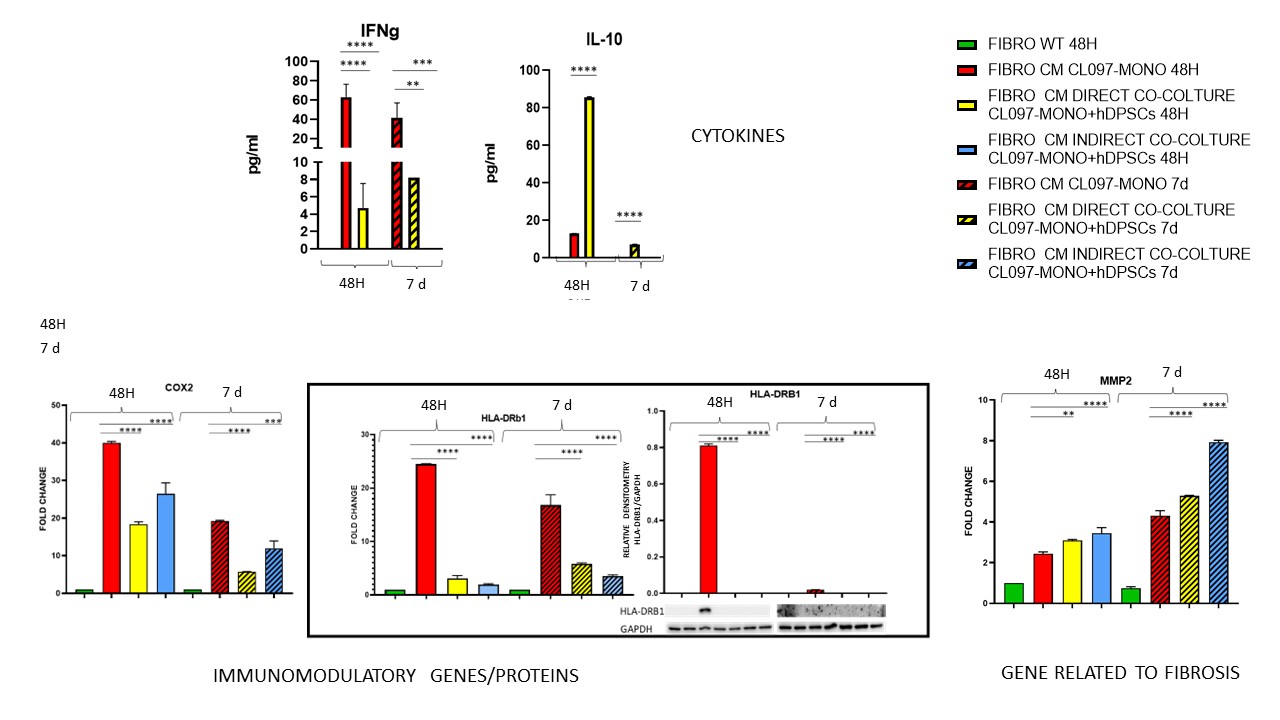
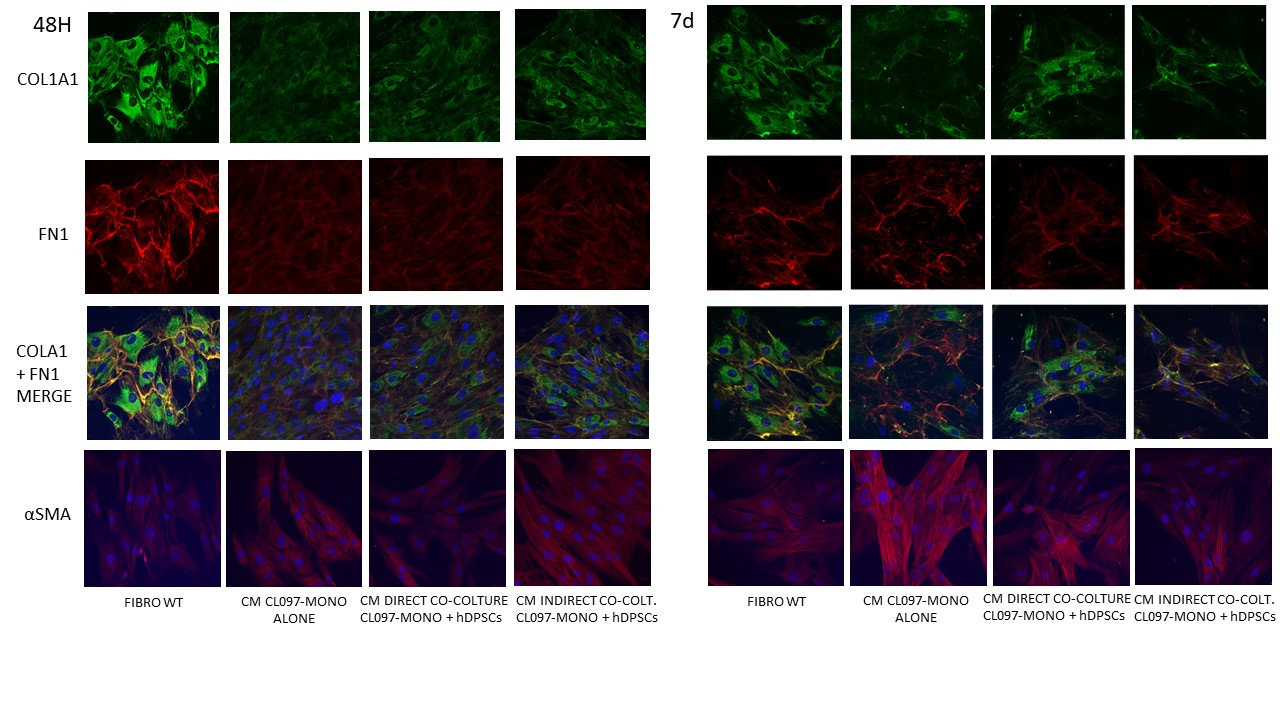
Treatment for 48 hours of fibroblasts with the conditioned medium of activated monocytes maintained in co-culture with hDPSCs induced a reduction in the expression of COX2 (p < 0,001) and HLA-DRB1 (p < 0,0001), an increase in the expression of MMP2 (p < 0,0001) and Col1A1 (p < 0,05), decrease in IFNγ (p < 0,001) and increase in IL-10 (p < 0,0001) secreted by fibroblasts, effect which persisted even after 7 days of treatment (FIG.2, 3).
Conclusion: The induction of a viral-type inflammatory environment by activated monocytes results in the early overexpression of proinflammatory cytokines and immunomodulatory genes and subsequently profibrotic genes in fibroblasts. The anti-inflammatory effects exerted by hDPSCs on monocytes are revealed in the indirect modulation of the immune response of fibroblasts by downregulation of HLA-DRB1, COX2, IFNγ and upregulation of IL-10. hDPSCs also exert antifibrotic and ECM remodeling effects by inhibition of αSMA and increased expression of Col1A1 and MMP2. The next step will be to evaluate the role of hDPSCs secretoma in inflammatory conditions to modulate the fibrotic process.
FIBROBLASTS TREATED FOR 48 HOURS OR 7 DAYS WITH CONDITIONED MEDIUM OF MONOCYTES ACTIVATED OR NOT WITH CL097
FIBROBLASTS TREATED FOR 48 HOURS OR 7 DAYS WITH CONDITIONED MEDIUM OBTAINED FROM DIRECT/INDIRECT CO-COLTURE OF CL097-MONOCYTES + hDPSCs
FIBROBLASTS TREATED FOR 48 HOURS OR 7 DAYS WITH CONDITIONED MEDIUM OF DIRECT/INDIRECT CO-COLTURE OF CL097-MONOCYTES + hDPSCs.
COL1A1, FN1 AND αSMA PROTEINS WERE REVEALED IN FIBROBLASTS BY IMMUNOFLUORESCENCE
To cite this abstract in AMA style:
Maccaferri M, Pisciotta A, Carnevale G, Salvarani C, PIGNATTI E. Effect of the Inflammatory Microenvironment Induced by Monocytes on Fibroblasts and Modulatory Action of Human Dental Pulp Stem Cells [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 9). https://acrabstracts.org/abstract/effect-of-the-inflammatory-microenvironment-induced-by-monocytes-on-fibroblasts-and-modulatory-action-of-human-dental-pulp-stem-cells/. Accessed .« Back to ACR Convergence 2023
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-the-inflammatory-microenvironment-induced-by-monocytes-on-fibroblasts-and-modulatory-action-of-human-dental-pulp-stem-cells/