Session Information
Session Type: Poster Session A
Session Time: 6:00PM-7:00PM
Background/Purpose: Few studies have evaluated the clinical characteristics and outcomes of youth with lupus nephritis (LN) treated with cyclophosphamide (CYC) who initially required kidney replacement therapy. CYC is partially renally cleared and the need for dose reduction with dialysis is controversial. Our study objectives were to summarize clinical characteristics of LN patients, describe treatment patterns, and compare the number of patients who required short-term dialysis versus those who developed end stage kidney disease (ESKD).
Methods: A multisite retrospective cohort study was conducted at 11 North American pediatric centers using electronic medical record data. Patients 6 to 22 years of age with active LN treated with either the lower dose EuroLupus (EL) or NIH CYC regimen from 7/1/2014 to 6/30/2021 were included. This subgroup analysis included patients who required dialysis at the time of CYC initiation (baseline). Patient data was collected at baseline, 3, 6, and 12 months. ESKD was defined as requiring dialysis therapy for >3 months. EL dosing was reviewed, and standard dosing was defined as 500 mg every 2 weeks for 6 doses, and any deviations from this dosing were recorded as modified. Baseline clinical characteristics and renal outcomes were summarized using descriptive statistics.
Results: Twenty patients met study inclusion criteria; of these, 15 (75%) received the EL regimen and 5 (25%) received the NIH regimen (Table 1). The average age at start of CYC during the study was 13.8 3.3 years. The cohort was predominantly female (75%), of diverse race/ethnicity, and publicly insured (60%). 80% of patients had new-onset LN while 20% had either relapsed or refractory disease. 95% of the cohort had proliferative LN and 30% had concurrent membranous disease. The mean cumulative dose of CYC was 5,823 mg 1,281 (NIH) and 2,681 mg 1,316 (EL). Of the 15 EL patients, 6 (40%) had modified dosing. Rituximab was given within 12 months of starting CYC to 40% of NIH regimen treated patients and 60% of EL treated patients. By 12 months, 3 patients (15%) had died, 4 (24%) met criteria for ESKD, and 13 (65%) had renal recovery off dialysis (Table 2). Of the patients with renal recovery, 11 of 13 (85%) were off before 3 months; 2 (15%) had an eGFR > 90 ml/min/1.73 m2 at 12 months (Table 2). Infections requiring hospitalization during the CYC treatment period occurred in 1 (20%) patient who received the NIH regimen and 7 (47%) patients who received the EL regimen. 5 of 8 (63%) patients hospitalized due to infection also received rituximab.
Conclusion: A majority of youth with proliferative LN requiring dialysis at baseline had new-onset LN and were treated with a lower dose CYC regimen (standard or modified EL). Over half of the patients received concurrent rituximab. Although most patients were able to discontinue dialysis, almost a quarter developed ESKD, and 3 patients died. Limitations include small sample size, lack of data on dialysis indication or dialysis type, and a follow-up duration of only 12 months. There is also practice variation regarding CYC dosing modification for patients on dialysis and optimal dosing is unclear. Future larger prospective studies are needed to better understand outcomes for this vulnerable population.
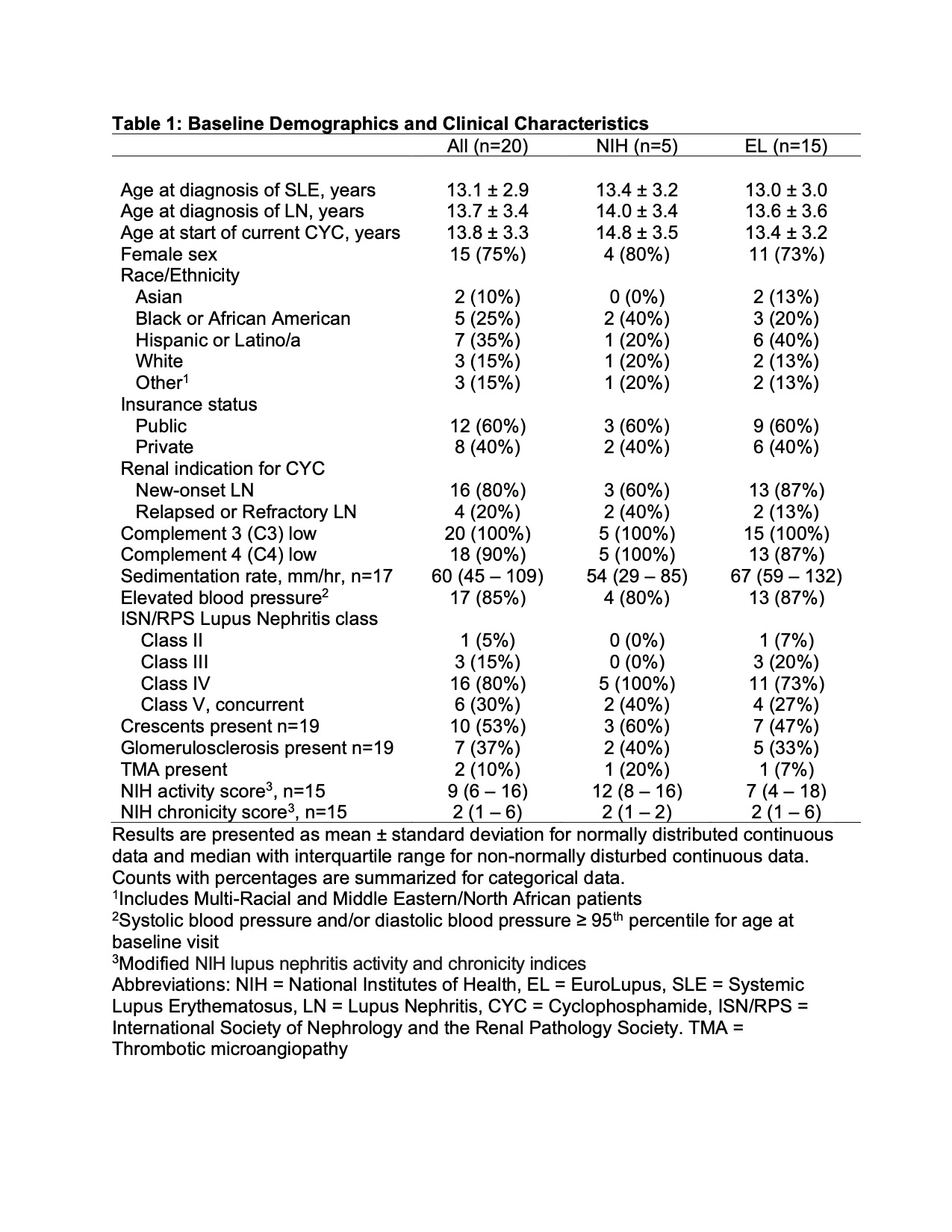 Table 1: Baseline Demographics and Clinical Characteristics
Table 1: Baseline Demographics and Clinical Characteristics
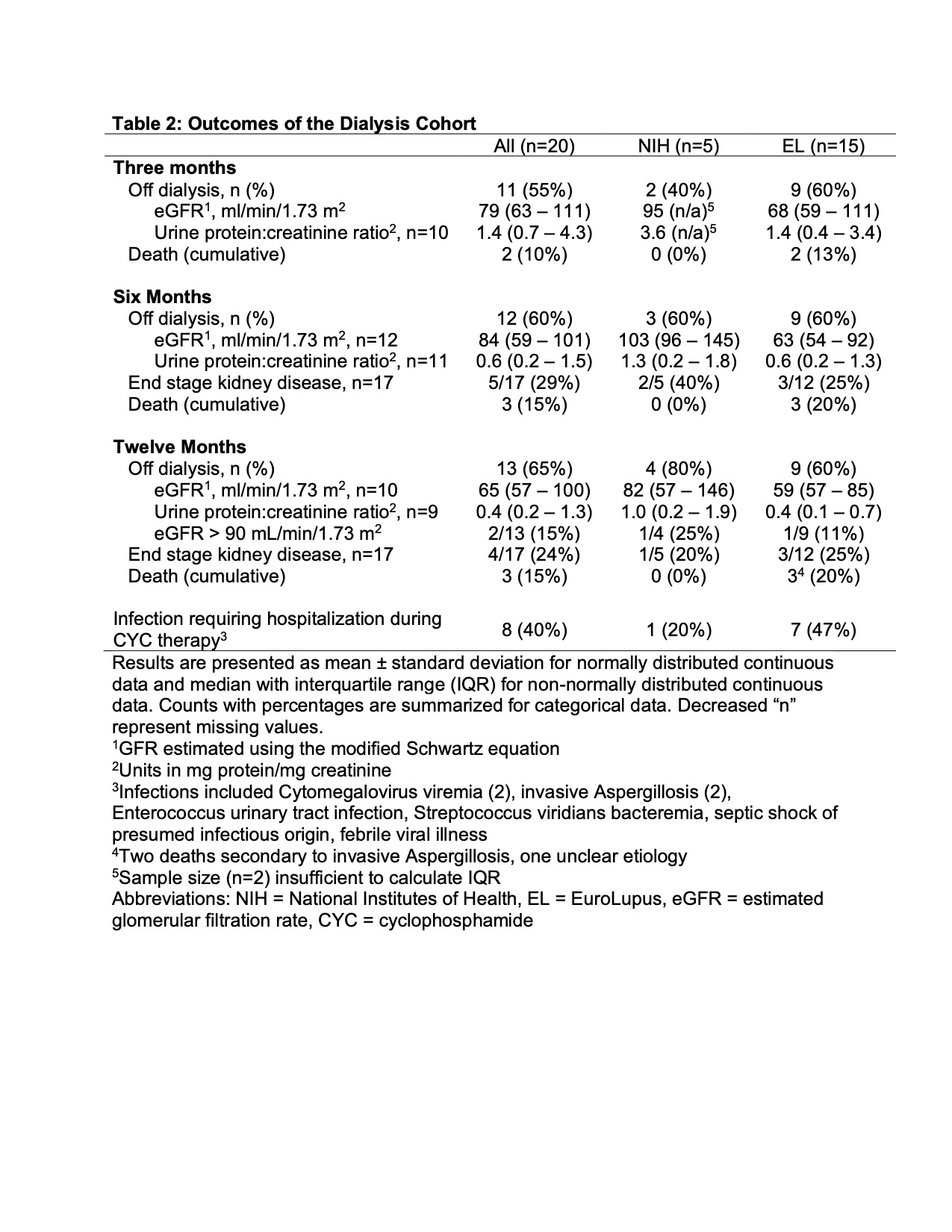 Table 2: Outcomes of the Dialysis Cohort
Table 2: Outcomes of the Dialysis Cohort
To cite this abstract in AMA style:
Wang C, Sadun R, Zhou W, Miller K, Palmer C, Ardoin S, Bacha C, Hause E, Hui-Yuen J, Ling N, Pereira M, Riebschleger M, Rouster-Stevens K, Sarkissian A, Shalen J, Soulsby W, Twilt M, Wu E, Lewandowski L, Wenderfer S, Cooper J. Clinical Characteristics and Outcomes of North American Youth with Lupus Nephritis Requiring Dialysis Treated with Cyclophosphamide [abstract]. Arthritis Rheumatol. 2023; 75 (suppl 4). https://acrabstracts.org/abstract/clinical-characteristics-and-outcomes-of-north-american-youth-with-lupus-nephritis-requiring-dialysis-treated-with-cyclophosphamide-2/. Accessed .« Back to 2023 Pediatric Rheumatology Symposium
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-characteristics-and-outcomes-of-north-american-youth-with-lupus-nephritis-requiring-dialysis-treated-with-cyclophosphamide-2/
