Session Information
Session Type: Poster Session D
Session Time: 1:00PM-3:00PM
Background/Purpose: Clinical and non-clinical forces contribute to real-world treatment effectiveness, yet most studies focus on efficacy in an idealized setting. To identify factors impacting clinical effectiveness, systematic chart reviews were initiated for patients with rheumatic diseases in care of the American Rheumatology Network (ARN). The result is a repository of data which combines information extracted from open text notes with fielded data in the EMR. Here we describe the process of creating the PIONEER repository for the purpose of informing future studies.
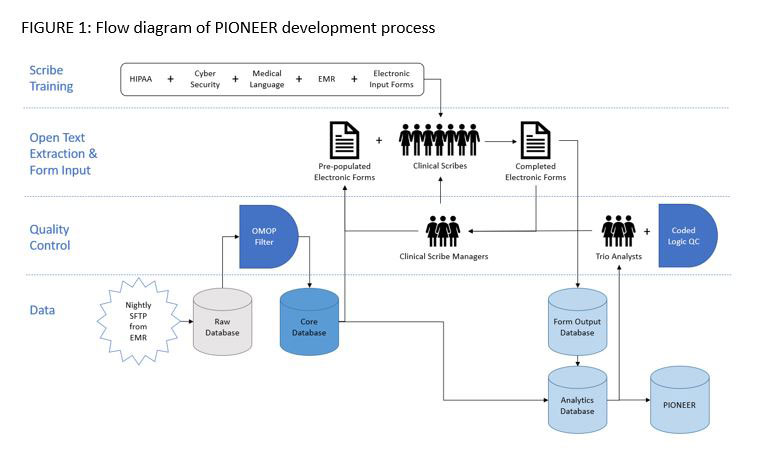
Methods: Data source: EMR lab, procedure, infusion, condition, and medical claims data and open text notes (office visits, infusions logs, provider-patient communications) generated in the care of patients by ARN, a network of independent practices with >200 rheumatologists across the US. Data in open text notes were extracted by clinically trained scribes who reviewed the full histories for each patient. [FIGURE 1] Each scribe received training for review, extraction, and data input processes, which were 21CFR11 and HIPAA compliant. Scribes confirmed or collected patient encounters, treatment history prior to ARN, treatment start dates or non-start reasons, treatment compliance and gaps, treatment discontinuation dates and discontinuation reasons. Electronic forms were coded to minimize data input errors and pre-populated with data received nightly from standardized EMR data files. Data collected in forms were subjected to logical checks to identify inputs requiring secondary review. Additionally, 1-2% of forms were randomly audited. Scribe workflow and data issue rates were assessed at least weekly and coupled to additional training and data reentry. Tertiary review of aggregate data was performed after combination of input form data with fielded EMR data.
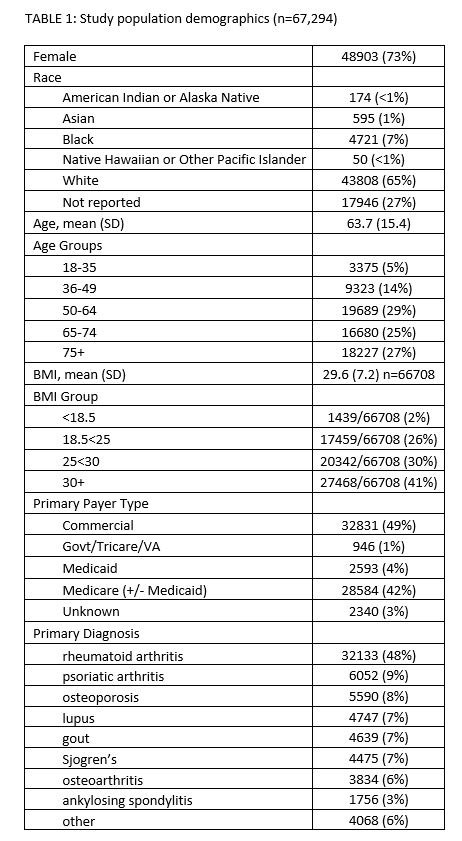
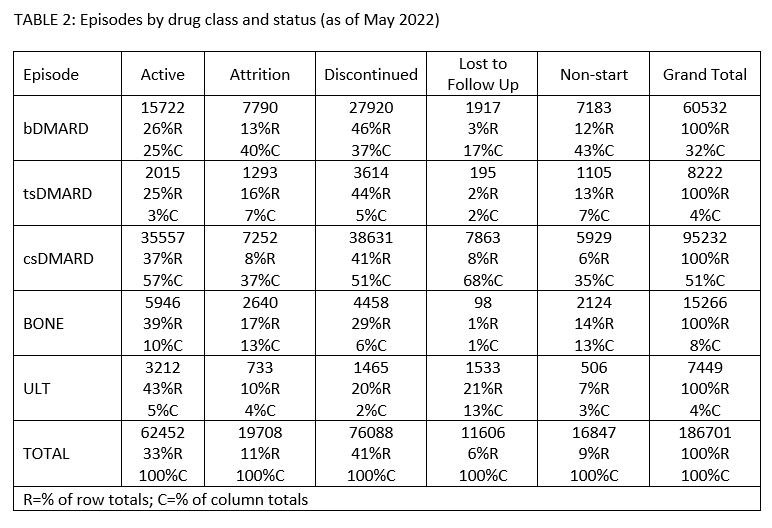
Results: As of May 2022, PIONEER-Rheumatology contained data for 172,593 patients; full chart reviews were completed for 67,294 patients (study population) prescribed conventional synthetic (cs), targeted synthetic (ts), or biologic (b) DMARDs, uric acid lowering therapies (ULT), and/or osteoporosis therapies (BONE). For study patients, clinical scribes reviewed 186,701 drug episodes and 695,716 office visits representing 316,652 observation years. 76% (51,317) and 50% (33,917) patients had >2 years and >4 years observation, respectively, and 77% (52,087) of patients were active in care in the preceding 36 months. Patient-reported state of residence includes all US States and DC though 98% (65,907) resided in AZ, FL, GA, KS, MO, SC, or TN. Study patients received care in one of 12 ARN practices across 32 Locations. Patient Demographics [TABLE 1]. Of 186,701 drug episodes, 9% (16,847) never started, 33% (62,452) were active, 11% (19,708) attrition (patient left practice or died), 6% (11,606) lost to follow-up, and 41% (76,088) discontinued due to a clinical reason. [TABLE 2]
Conclusion: The PIONEER data repository uniquely combines open text information with fielded data residing in the EMR and will enable better understanding of patient-important outcomes that may not be adequately represented in commonly used disease assessments.
To cite this abstract in AMA style:
Helfgott S, Huston K, singh J, Soloman N, Broestl J, Cox K, Milligan K, Edgerton C. Development of the Patient-Important Outcomes Data Repository (PIONEER) for Rheumatic Diseases; An Enhanced Database Combining Electronic Medical Data with Insight from Chart Reviews [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/development-of-the-patient-important-outcomes-data-repository-pioneer-for-rheumatic-diseases-an-enhanced-database-combining-electronic-medical-data-with-insight-from-chart-reviews/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-the-patient-important-outcomes-data-repository-pioneer-for-rheumatic-diseases-an-enhanced-database-combining-electronic-medical-data-with-insight-from-chart-reviews/