Session Information
Session Type: Abstract Session
Session Time: 10:30AM-11:30AM
Background/Purpose: To estimate the frequency of rheumatic toxicities of immune checkpoint inhibitors (ICI) presenting as de novo or exacerbations of pre-existing rheumatic disease in patients with advanced melanoma. To explore the effect of rheumatic toxicities on ICI treatment outcomes and identify predictors of favourable tumour responses.
Methods: A sub-analysis of a single-centre observational study examining the epidemiology, risk factors and clinical course of rheumatic toxicities in patients with stage III or IV melanoma receiving all available ICI therapies. Patients were recruited upon completion of a for-purpose questionnaire designed to capture rheumatic symptoms and risk factors and were followed up at 12-months. Clinical details were extracted from patients’ medical records over this period. Tumour objective response rates (ORR) were compared between patients with versus without both patient-reported and clinician-recorded rheumatic toxicities. Association between tumour response and relevant pre-morbid, melanoma and treatment related factors were explored using chi squared tests of heterogeneity and univariate analysis. Models for prediction of favourable tumour response were generated using binary logistic regression.
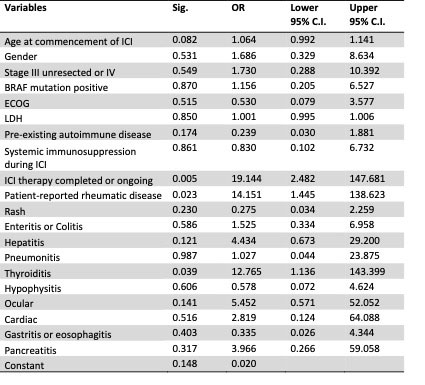
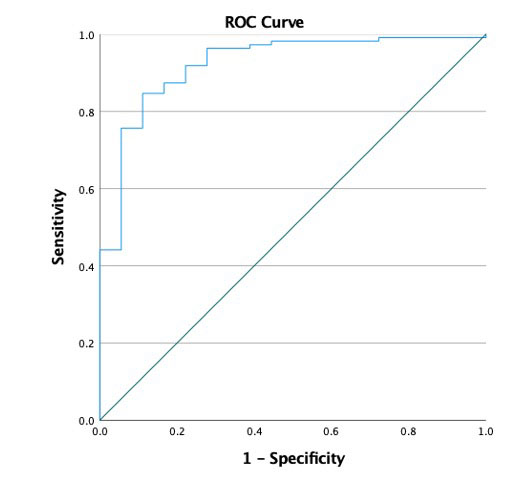
Results: Amongst 147 eligible patients, the prevalence of de novo or exacerbations of pre-existing immune and non-immune mediated rheumatic disease according to initial questionnaire responses was 32.5%, and 21% at 12 months. The incidences of clinician-documented rheumatic syndromes such as arthralgia, inflammatory arthritis and PMR-like syndrome were 39.5%, 5.4% and 3.4% respectively. The overall ORR in this cohort was 87.1%, derived from 128 of 147 patients, with complete response, partial response, stable disease and disease free (adjuvant therapy) tumour responses occurring in 31.3%, 29.9%, 6.1% and 19% of patients respectively. There was a statistically significantly higher ORR in patients with versus without clinician-documented rheumatic disease, ie 94% versus 78.1% (χ2(1) = 8.067, Fisher exact test p = 0.006), with a 4 fold improvement in odds of obtaining an objective response (OR = 4.368, 95% CI 1.428 – 12.867). Binary logistic regression identified rheumatic toxicities (OR = 14.151, 95% CI 1.445-138.623) followed by thyroiditis (OR = 12.756, 95% CI 1.136-143.399) as statistically significant predictors of favourable tumour response (Table 1) and this model was found to have 92% accuracy (Figure 1). Treatment with immunosuppression did not show a detrimental effect on tumour response (OR 1.129, 95% CI 0.183–6.956)(Table 1).
Conclusion: Rheumatic toxicity in patients with advanced melanoma treated with ICIs is associated with favourable tumour responses when evaluated in the context of other potential predictors including other organ-specific immune related adverse events. Treatment with immunosuppression for any immune-related adverse event does not negatively affect tumour response.
(a) Under the nonparametric assumption
(b) Null hypothesis: true area = 0.5
To cite this abstract in AMA style:
Bruce A, Menzies A, Long G, Fernandes B, Joshua F. Rheumatic Toxicities of Immune Checkpoint Inhibitors Predict Favourable Tumour Responses in Patients with Advanced Melanoma [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/rheumatic-toxicities-of-immune-checkpoint-inhibitors-predict-favourable-tumour-responses-in-patients-with-advanced-melanoma/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatic-toxicities-of-immune-checkpoint-inhibitors-predict-favourable-tumour-responses-in-patients-with-advanced-melanoma/