Session Information
Date: Sunday, November 13, 2022
Title: SLE – Treatment Poster II
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Enpatoran, a novel, highly selective and potent dual toll-like receptor (TLR) 7 and TLR8 inhibitor, is in development for the treatment of autoimmune disorders including cutaneous and systemic lupus erythematosus (CLE/SLE). A first-in-human study in healthy participants (PTPs) has shown that enpatoran is well-tolerated, with a linear pharmacokinetic (PK) profile. We compared the PK parameters, safety, and tolerability of single ascending oral doses of enpatoran in a Phase I study in Japanese and Caucasian PTPs and explored a potential PK/pharmacodynamic (PD) relationship.
Methods: A single-center, open-label, sequential dose group study enrolled healthy Japanese and Caucasian PTPs into three dose cohorts. Each Caucasian PTP was matched by body weight (±20%), height (±15%) and sex to a Japanese PTP. PTPs received a single enpatoran dose of 100 mg, 200 mg or 300 mg as a film-coated tablet under fasting conditions. PK parameters, (maximum plasma concentration [Cmax]; area under the plasma concentration–time curve (AUC) from time 0 to infinity [AUC0-inf]; AUC from time 0 to the last sampling time [AUC0-tlast]) determined using noncompartmental analysis, were estimated post-dose (Days 1–3). Safety was assessed from Day -1 to 8. PK (exposure) between the two ethnic groups was compared using an analysis of covariance (ANCOVA) model including ethnic group, natural log-transformed dose, and ethnic group by natural log dose interaction. Stimulated (using the TLR7/8 agonist, R848) and unstimulated ex vivo cytokine secretion (PD) was assessed pre-/post-dose. A panel of cytokines was analyzed by multiplex immunoassay; interleukin 6 (IL-6) was considered the primary PD biomarker.
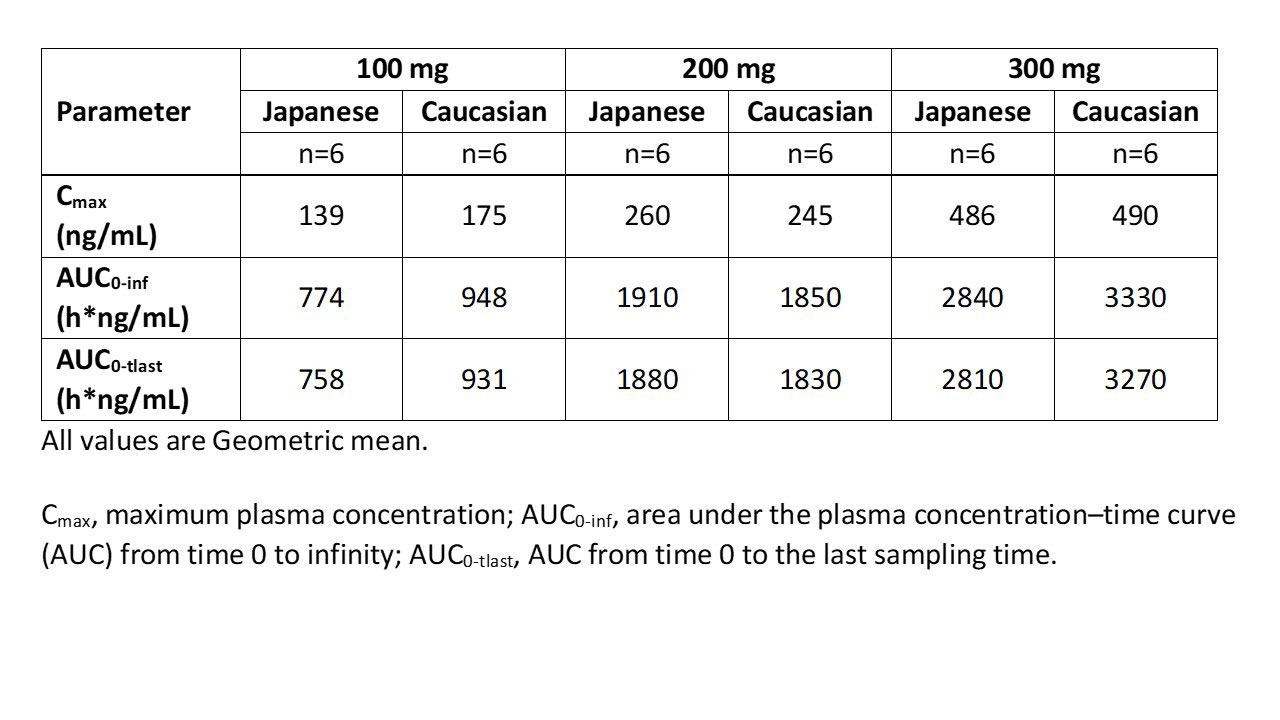
Results: The study included 36 male PTPs (18 Japanese, 18 Caucasian) with a mean (±SD) age of 35.1 (±10.8) years and body mass index of 23.1 (±2.1) kg/m2. Each dose group included six Japanese and six Caucasian PTPs. Geometric mean enpatoran plasma exposure parameters were consistent between the two ethnic groups for each dose (Table) and indicated dose proportionality. ANCOVA modelling demonstrated comparable exposure between the two groups (geometric least square mean ratio [Japanese/Caucasian; 90% CI] of Cmax: 0.9409 [0.7855–1.1270]; AUC0-inf: 0.8959 [0.7497–1.0704] and AUC0-tlast: 0.8963 [0.7511–1.0695]).
Treatment-emergent adverse events (TEAEs) were observed in six Japanese (n=0, 100 mg; n=3, 200 mg; n=3, 300 mg) and four Caucasian (n=1, 100 mg; n=0, 200 mg; n=3, 300 mg) PTPs; none were serious. There were no deaths, withdrawals, or early terminations due to TEAEs. Maximal inhibition of cytokine release was observed at 2 hours post-dose (IL-6: mean ≥99%). High inhibition levels were sustained through 24 hours in a dose-dependent manner (IL-6: mean ~76–97%). The pattern of cytokine release inhibition was consistent across doses and ethnic groups.
Conclusion: PK, PD, and safety results were comparable between healthy Japanese and Caucasian PTPs across a range of single oral enpatoran doses, supporting the inclusion of Asian PTPs in future global enpatoran Phase II studies, including WILLOW (NCT05162586), a basket proof-of-concept and dose finding SLE/CLE study, initiated in 2022.
To cite this abstract in AMA style:
Gopalakrishnan S, Krebs-Brown A, Nogueira Filho M, Kuroki Y, Bachmann A, Becker A, Schippers F, Fluck M, Yalkinoglu Ö, Kleinmond C, Chitkara D, Vazquez Mateo C, Roy S, Klopp-Schulze L. Safety, Pharmacokinetics, and Pharmacodynamics of Single Oral Enpatoran Doses in a Phase I Study of Healthy Japanese and Caucasian Participants: Feasibility of Including Asian Participants in a Phase II Study of Enpatoran [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/safety-pharmacokinetics-and-pharmacodynamics-of-single-oral-enpatoran-doses-in-a-phase-i-study-of-healthy-japanese-and-caucasian-participants-feasibility-of-including-asian-participants-in-a-phase/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-pharmacokinetics-and-pharmacodynamics-of-single-oral-enpatoran-doses-in-a-phase-i-study-of-healthy-japanese-and-caucasian-participants-feasibility-of-including-asian-participants-in-a-phase/