Session Information
Session Type: Poster Session B
Session Time: 9:00AM-10:30AM
Background/Purpose: Treatment with a combination of immune checkpoint inhibitors (ICI) has promising outcomes in many tumor types but carries higher adverse event risk than ICI monotherapy. Also, patients with pre-existing autoimmune disease (AID) have largely been excluded from ICI clinical trials due to concern for pre-existing AID flare or immune-related adverse events (irAEs). This is the first study to analyze safety and effectiveness of ICI combination versus monotherapy for this at-risk population.
Methods: We conducted a multi-center retrospective study in patients with pre-existing AIDs receiving ICIs (i.e., anti-programmed cell death protein 1 (PD-1) single-agent (monotherapy) or ICI combination). Primary endpoints included the time to occurrence of any-type ICI AE (irAE or AID flare), time to irAEs and time to AID flares in the presence of the competing risk of death with progression free survival (PFS, time to progression or death) and overall survival (OS) as secondary endpoints. We used Fine-Gray models and Cox regression models to investigate the factors associated with these endpoints.
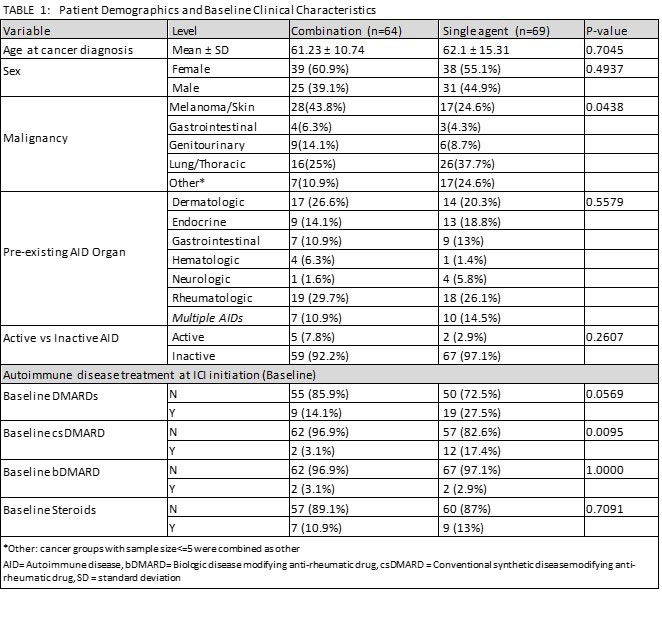
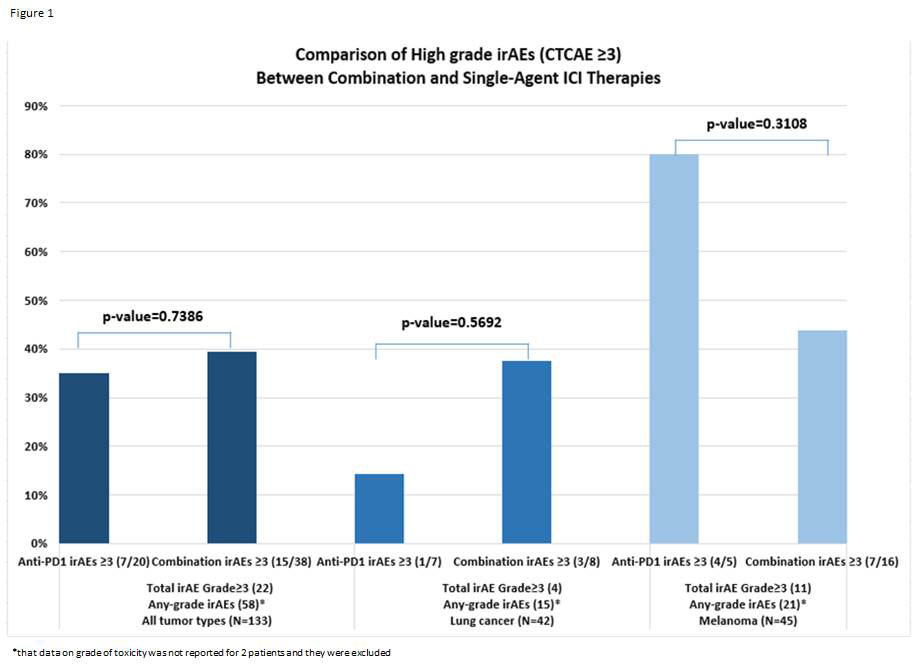
Results: 133 patients with pre-existing AID who received ICIs were identified: 69 (52%) monotherapy and 64 (48%) combination (Table 1). About half the patients had melanoma (44%) and 25% had lung cancer. Rheumatic (34%) or dermatologic (22%) pre-existing AIDs were the most common. Most patients (95%) had controlled autoimmune disease at ICI start. Six of 7 patients with active AID at baseline experienced some AE. Patients receiving baseline DMARD(s) were more likely to experience an AE (95%CI 1.079-2.996, p=0.024). The cumulative incidence of irAEs was higher for ICI combination compared to monotherapy (subdistribution hazard ratio (sHR) 2.28, 95%CI 1.36-3.84), adjusting for age at malignancy, but there was no significant difference between rate of high-grade toxicity for patients treated with ICI combination versus monotherapy (See Figure 1). On subgroup analysis for patients with melanoma or lung cancer, the cumulative incidence of irAEs or AID flares were not statistically different between treatment groups. PFS was longer (but not statistically significant) for combination therapy for any tumor type compared to single agent (median 12.3mo, 95%CI 5.0-23.2 versus 7.3mo, 95%CI 5.2-11.3, p=0.116). Similar trend was noted for PFS for melanoma (median 23.2mo combination vs. 14.0mo monotherapy, p=0.4237), while the opposite relation was noted for lung cancer subgroup (4.4mo combination vs. 7.1mo monotherapy, p=0.2933).
Conclusion: Efficacy of ICI combination versus monotherapy was not statistically significant and so still remains unclear in this patient population, but there was no significant difference in rates of high-grade toxicity between the two cohorts. Our data supports the notion that patients with pre-existing AIDs should not be indiscriminately precluded from getting ICI combination. Our results provide guidance for future prospective clinical trials studying combination therapy for subgroups of this at-risk population.
To cite this abstract in AMA style:
Reid P, Sandigursky S, Lopez-Olivo M, Song J, Safa H, Cytryn S, Buni M, Pavlick A, Krogsgaard, PhD M, Abu-Shawer O, Altan M, Weber J, Suarez-Almazor M, Diab A, Abdel-Wahab N. Safety and Effectiveness of Immune Checkpoint Inhibitors Combination versus Single Agent Therapy in Patients with Pre-existing Autoimmune Diseases [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/safety-and-effectiveness-of-immune-checkpoint-inhibitors-combination-versus-single-agent-therapy-in-patients-with-pre-existing-autoimmune-diseases/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-and-effectiveness-of-immune-checkpoint-inhibitors-combination-versus-single-agent-therapy-in-patients-with-pre-existing-autoimmune-diseases/