Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Olokizumab (OKZ), an IL-6 ligand inhibitor, demonstrated significant improvements in signs and symptoms of RA vs placebo (PL) and non-inferiority to adalimumab (ADA).1-2 Patients (pts) who completed core phase III studies, were enrolled in an open-label extension study (OLE) (ClinicalTrials.gov number, NCT03120949). Here we report long-term safety and efficacy results of treatment with OKZ in combination with MTX.
Methods: During the OLE, patients (pts) continued OKZ 64 mg q2w or q4w, and those who were on PL or ADA were randomized to OKZ 64 mg q2w or q4w.
Adverse events (AE) were assessed through 82 weeks (Wk) of the OLE treatment and additional 20 weeks of safety follow-up. All AEs were analyzed using descriptive statistics.
Efficacy assessments in observed pts included proportions of pts who achieved ACR20/50/70 responses, simplified disease activity index (SDAI) ≤3.3, improvement in HAQ-DI ≥0.22, mean change in clinical disease activity index (CDAI), and DAS28-CRP, based on descriptive statistics.
Results: Of 2105 eligible pts enrolled, 1709 (81.2%) completed the OLE treatment period and 394 (18.7%) discontinued treatment: 180 (8.6%) due to AE, 158 (7.5%) due to pts decision, 9 (0.4%) due to lack of efficacy and 47 (2.2%) due to various reasons including protocol violation, pregnancy, lost to follow-up, discontinuation by sponsor, study terminated by sponsor or investigators and other reasons.
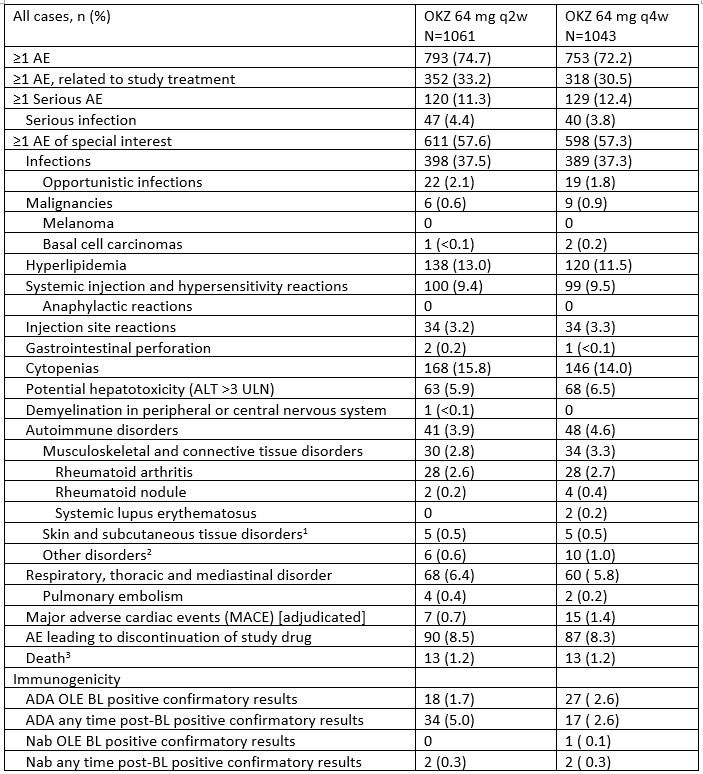
Treatment emergent AEs occurred in 1546 (73.5%) pts, the most common AEs 809 pts (38.5%) were infections. The most common AEs leading to treatment discontinuation were infections (53 [2.5%]), laboratory investigations (43 [2.0%]), including alanine aminotransferase increase (21 [1%]), blood and lymphatic system disorders (13 [0.6%]), and cytopenias (leukopenia = 0.2%, lymphopenia = 0.1%, others (neutropenia, thrombocytopenia) < 0.1%) (Table).
Serious AEs were reported by 249 (11.8%) pts, serious infections – by 87 (4.1%) pts.
Deaths occurred in 1.2% of pts, equally distributed between the two groups with the most common causes included infections – 0.3%, general disorders and administration site conditions – 0.2% (including death – 2 pts, sudden death – 2 pts, sudden cardiac death – 1 pt) and cardiac disorders, neoplasms benign, malignant and unspecified and respiratory, thoracic and mediastinal disorders – 0.1% each.
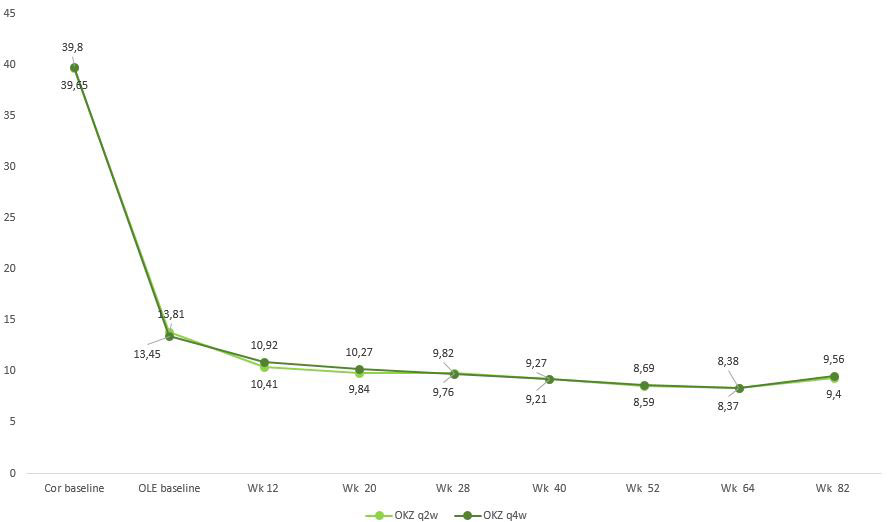
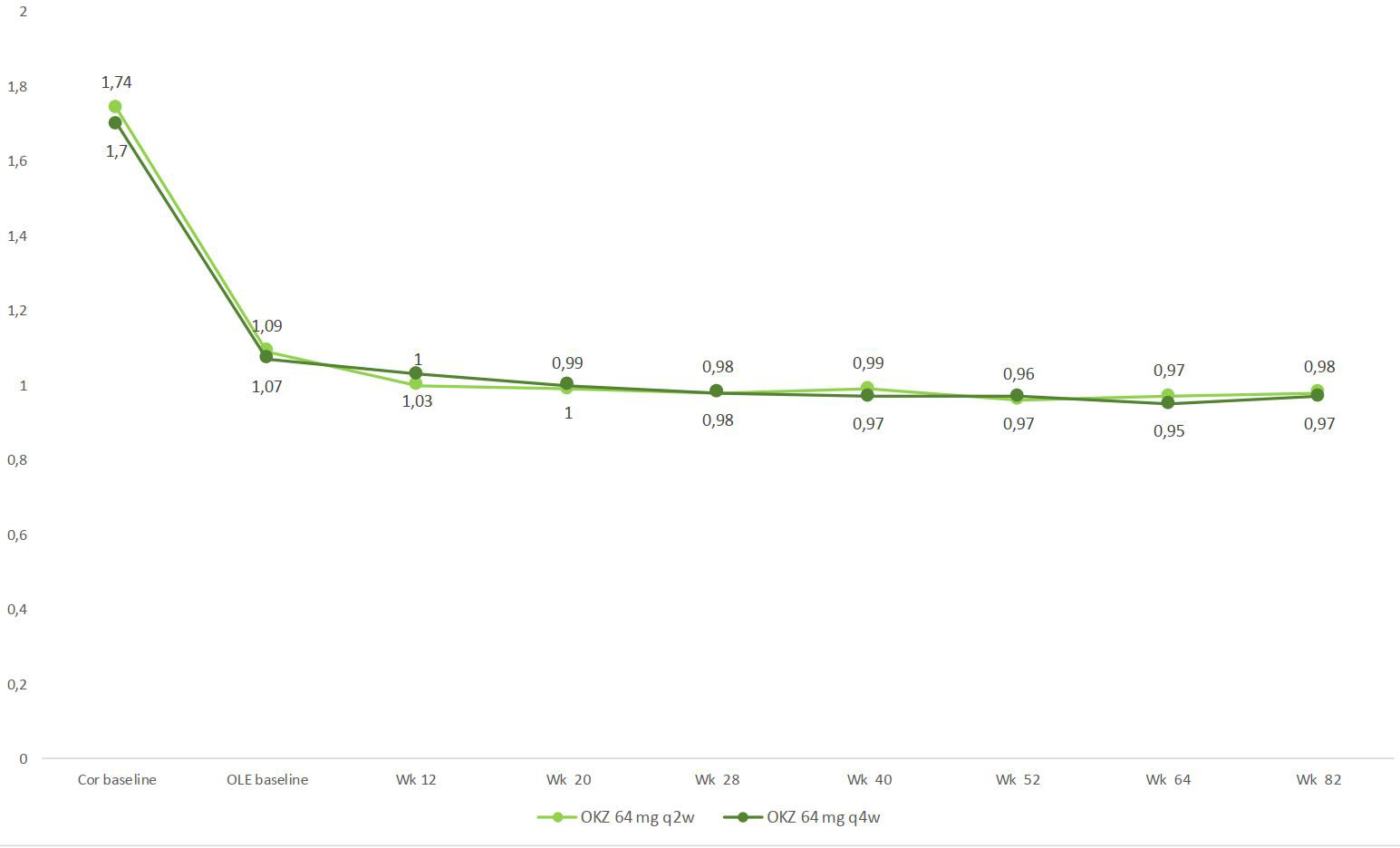
Efficacy of OKZ was maintained throughout the study (Fig. 1, 2) and pts who switched from PL or ADA showed the same level of improvement as those who were treated with OKZ in the core studies (data not shown).
There were no apparent differences in safety and efficacy outcomes between OKZ arms. Major adverse cardiac events (MACE) occurred in 7 (0.7%) pts in OKZ q2w group and in 15 (1.4%) pts in OKZ q4w group (Table).
Incidence rate of anti-drug antibodies, neutralizing antibodies, malignancies, nonmelanoma skin cancer and venous thromboembolism are reported (Table).
Conclusion:
OKZ was generally safe and well-tolerated, consistent with the results of previously reported studies for OKZ and other IL-6 inhibitors. Efficacy of OKZ was maintained through Wk 82 in pts who continued in the OLE study with low discontinuation rates.
R
Abbreviations: ADA, antidrug antibodies; AE, adverse event that occurred after the first dose of the open-label (OLE) study treatment; ALT, alanine aminotransferase; BL, baseline; n, number of subjects; Nab, neutralizing antibodies; q2w, every 2 weeks; q4w, every 4 weeks; ULN, upper limit normal; %, percentage of subjects calculated relative to the total number (N) of subjects in the population.
Footnotes: 1 – Psoriasis, cutaneous vasculitis, alopecia areata, hypersensitivity vasculitis, pyoderma gangrenosum, vasculitic rash, vitiligo;
2 – Chronic gastritis, ulcerative keratitis, ocular myasthenia, vasculitis, rheumatoid vasculitis, autoimmune thyroiditis, type 1 diabetes mellitus, rheumatoid lung.
3 – AEs leading to death by System Organ Class: infections and infestations 7 (0.3%), general disorders and administration site conditions 5 (0.2%), cardiac disorders 3 (0.1%), neoplasms benign, malignant and unspecified (CNS neoplasm, metastatic neoplasm, pancreatic carcinoma metastatic) 3 (0.1%).
Abbreviations: CDAI, clinical disease activity index; Cor, phase III randomized double-blind clinical trials; OLE, Open-Label Extension; q2w, every 2 weeks; q4w, every 4 weeks; Wk, week.
Footnote: Wk 82, includes patients who stopped treatment early at any time during the study.
Abbreviations: Cor, phase III randomized double-blind clinical trials; OLE, open-label extension; q2w, every 2 weeks; q4w, every 4 weeks.
Footnote: Wk 82, includes patients who stopped treatment early at any time during the study.
References:
1. Nasonov E, et al. Ann Rheum Dis. 2021; 0:1–11
2. Feist E, et al. Ann Rheum Dis. 2022; 81:593
To cite this abstract in AMA style:
Feist E, Nasonov E, Luggen M, Fatenejad S, Grishin S, Samsonov M, Fleischmann R. Long-Term Safety and Efficacy of Olokizumab in Patients with Rheumatoid Arthritis – Results of an Open-Label Extension Study [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-olokizumab-in-patients-with-rheumatoid-arthritis-results-of-an-open-label-extension-study/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/long-term-safety-and-efficacy-of-olokizumab-in-patients-with-rheumatoid-arthritis-results-of-an-open-label-extension-study/