Session Information
Date: Saturday, November 12, 2022
Title: RA – Treatment Poster I
Session Type: Poster Session A
Session Time: 1:00PM-3:00PM
Background/Purpose: Tofacitinib is an oral Janus kinase inhibitor for the treatment of rheumatoid arthritis. ESCALATE-RA is the first prospective, non-interventional study with tofacitinib in Germany.
Methods: Enrollment of the planned 1500 patients took place Germany-wide at 88 recruiting sites between 2017 and 2021. Adult patients eligible for tofacitinib therapy are documented quarterly in a standardized manner from the first tofacitinib intake up to 24 months and remain within the study even after switching to another disease-modifying drug (DMARD) or combination of DMARDs. This third interim analysis (cut-off date 31 Jan 2022) evaluates patients who reached the final visit M24 after 24 months. Missing observations were not taken into account. Since this is an ongoing study, small changes in numbers may occur after the final data quality checks.
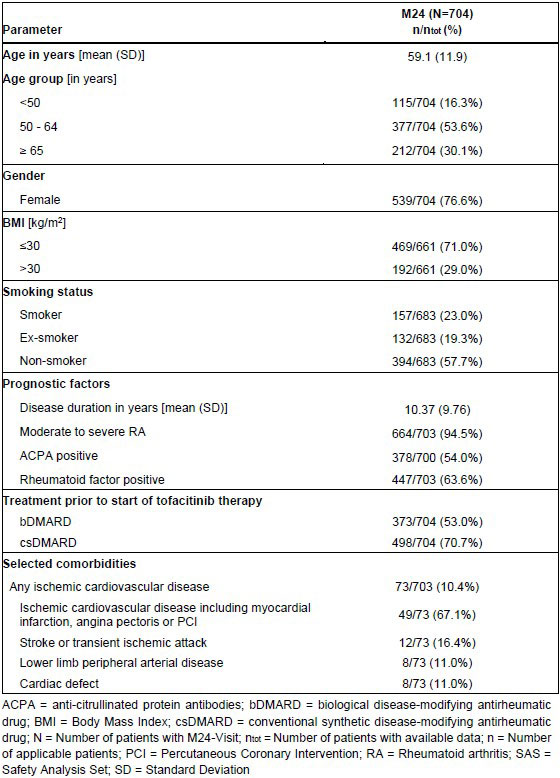
Results: At data cut-off, 1521 patients were enrolled, of whom 704 (51.4%) patients reached visit M24. Mean age was 59 years; approx. three quarters of patients were female. Other baseline characteristics are depicted in Table 1. Cardiovascular disease was present at baseline in 10.4% of patients: of these, 67.1% had ischemic cardiovascular disease, 16.4% had stroke or transient ischemic attack, and 11.0% each had lower limb peripheral arterial disease or cardiac defect (Table 1).
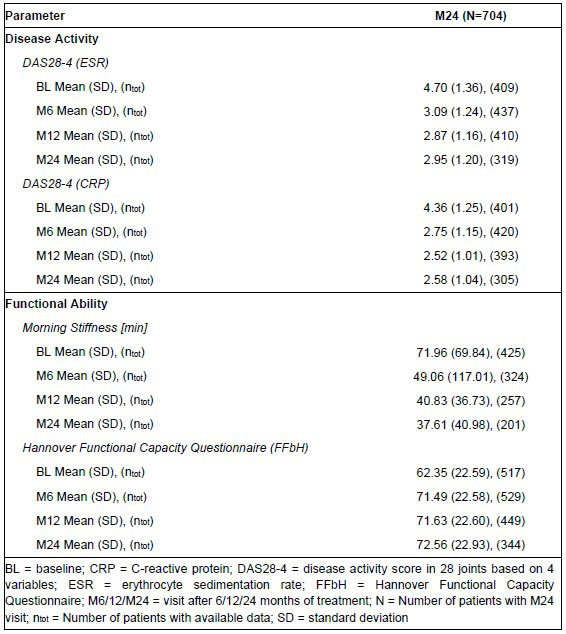
A reduction of disease activity and morning stiffness was observed over time while functional capacity according to FFbH (Hannover Functional Capacity Questionnaire, German short questionnaire for the assessment of functional capacity in the context of basic everyday activities (range: 0-100% functional capacity) [1]) increased (Table 2). Furthermore, an improvement of self-reported quality of life based on EQ-5D-3L and Functional Assessment of Chronic Illness Therapy (FACIT) Fatigue Scale was observed over time.
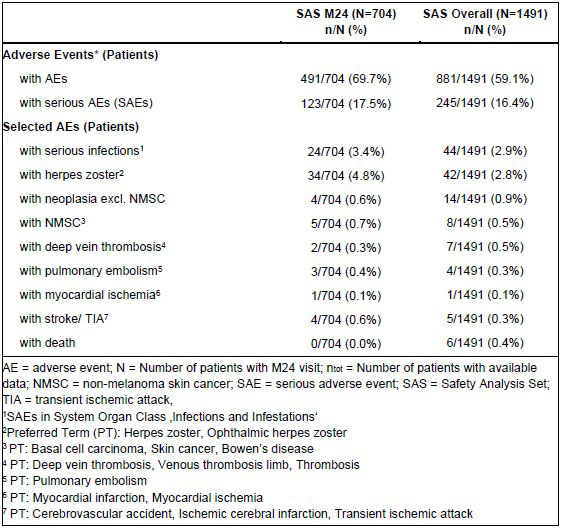
In the M24 population during 24 months of observation, 69.7% of patients experienced adverse events (AEs) and 17.5% experienced serious AEs. Apart from lack of efficacy (11.8%), the most common treatment-related AEs were influenza-like illness (4.4%), herpes zoster (4.8%), and nasopharyngitis (3.8%). Approximately one-third of patients discontinued tofacitinib treatment due to an AE. In the Overall Population, 6 out of 1491 patients (0.4%) died (Table 3).
Conclusion: This third interim analysis confirms effectiveness and increased quality of life with tofacitinib therapy. Safety results were consistent with the known safety profile of tofacitinib. These results are in line with those of the second interim analysis.
To cite this abstract in AMA style:
Krüger K, Prothmann U, Klopsch T, Behmer O, Hsieh M, Jobst J, Klaus P, Meng T. Effectiveness and Safety of Tofacitinib Treatment in Adult Patients with Rheumatoid Arthritis Under Routine Clinical Care: Third Interim Analysis of a German Non-Interventional, Prospective, Multicenter Study (ESCALATE-RA) [abstract]. Arthritis Rheumatol. 2022; 74 (suppl 9). https://acrabstracts.org/abstract/effectiveness-and-safety-of-tofacitinib-treatment-in-adult-patients-with-rheumatoid-arthritis-under-routine-clinical-care-third-interim-analysis-of-a-german-non-interventional-prospective-multicent/. Accessed .« Back to ACR Convergence 2022
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effectiveness-and-safety-of-tofacitinib-treatment-in-adult-patients-with-rheumatoid-arthritis-under-routine-clinical-care-third-interim-analysis-of-a-german-non-interventional-prospective-multicent/