Background/Purpose: Anti-neutrophil cytoplasmic autoantibody (ANCA)-associated vasculitis is a life- or organ-threatening condition in which patients experience severe inflammation of small blood vessels; renal disease is common and confers a high risk for end-stage renal disease and death. Efficacy and safety results from a phase 3 trial evaluating avacopan, an oral selective C5a receptor inhibitor, in patients with ANCA-associated vasculitis have been previously reported. The effect of treatment with avacopan on renal function is detailed here.
Methods: ADVOCATE was a phase 3, randomized, double-blind, controlled clinical study. All patients were randomized to receive prednisone taper or avacopan on a background of either cyclophosphamide (followed by azathioprine) or rituximab. Primary efficacy endpoints were the percent of patients achieving disease remission at Week 26 and sustained remission at Week 52. Secondary objectives included evaluation of kidney function, specifically the change from entry (baseline) through to Week 52 in estimated glomerular filtration rate (eGFR) and urinary albumin:creatinine ratio.
Results: In ADVOCATE, 268/330 (81%) patients had renal disease at baseline. Average eGFR at baseline in patients with renal disease was 44.6 and 45.6 mL/min/1.73 m2 in the avacopan (n=134) and prednisone (n=134) groups, respectively. eGFR improved more in the avacopan group compared to the prednisone group. At Week 52, the least squares mean (LSM) increase in eGFR in the avacopan and prednisone groups was 7.3 and 4.1 mL/min/1.73 m2 (p=0.03), respectively (Figure 1).
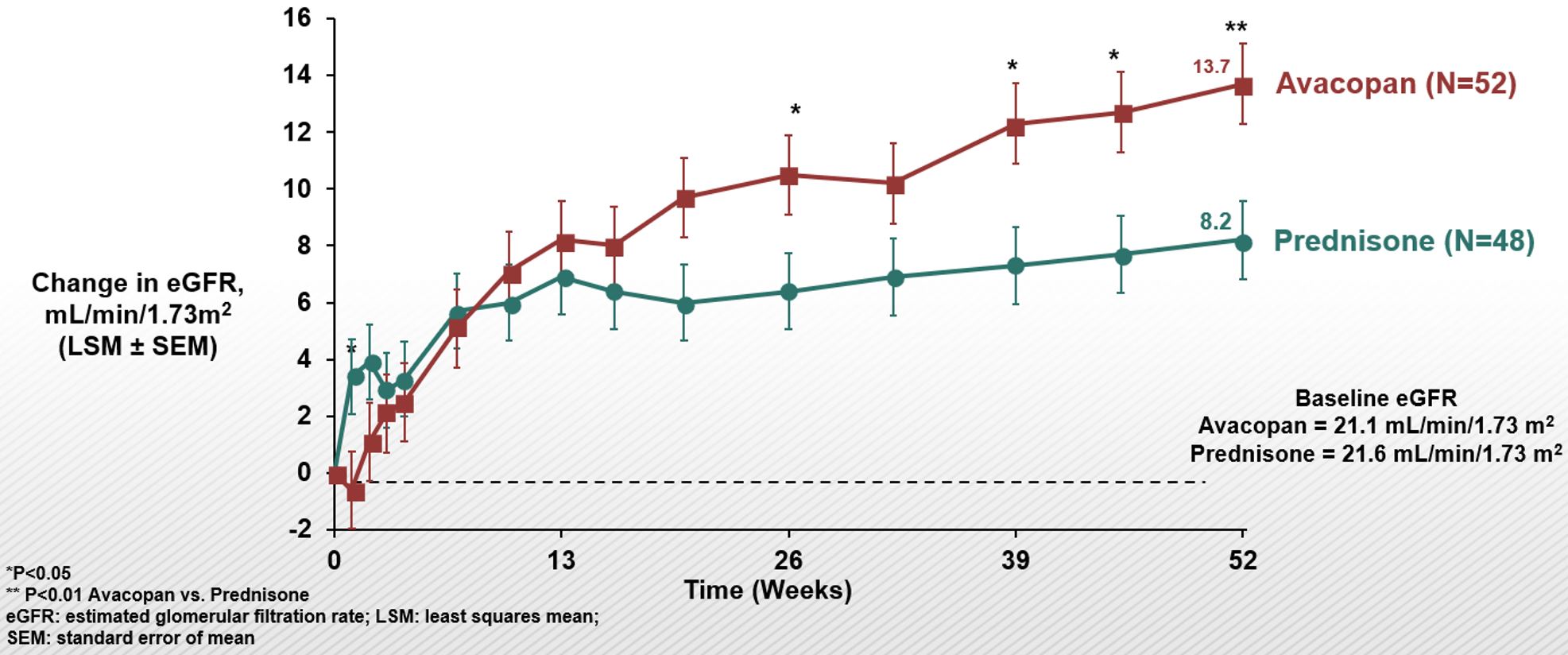
At Week 52, in patients with baseline eGFR < 60 mL/min/1.73 m2 , U-RBC ≥10/hpf, and U-ACR ≥0.3 g/g the least squares mean (LSM) increase in eGFR was 12.7 (avacopan group) vs. 7.1 mL/min/1.73 m2 (prednisone group) (p< 0.01) (Figure 2). Improvement in eGFR with avacopan was most prominent in patients with baseline eGFR < 30 mL/min/1.73 m2, for whom by week 52 the least squares mean (LSM) increase in eGFR was 13.7 (avacopan group) vs. 8.2 mL/min/1.73 m2 (prednisone group) (p< 0.01) (Figure 3).
Among patients with Stage 4 chronic kidney disease at baseline: in the avacopan group (n=52), 26 (50%) improved 1 stage and 3 (5.8%) improved 2 stages; in the prednisone group (n=48), 21 (44%) improved 1 stage and 1 (2.1%) improved 2 stages (p=0.32). Avacopan was also associated with more rapid reduction in proteinuria. The largest difference in improvement in urinary albumin:creatine ratio was 40% at 4 weeks of treatment; the LSM±SEM change from baseline was –40±9.5 in the avacopan group compared to 0±9.3 in the prednisone group.
Conclusion: Patients with ANCA-associated vasculitis in the avacopan group had greater recovery of kidney function compared to patients in the prednisone group, especially among patients with chronic kidney disease Stage 4 and those with eGFR < 60 mL/min/1.73 m2 and urinary abnormalities at baseline.
To cite this abstract in AMA style:
Jayne D, Merkel P, Bruchfeld A, Geetha D, Karras A, Niles J, Bekker P. Effect of Avacopan, a Selective C5a Receptor Inhibitor, on Kidney Function in Patients with ANCA-Associated Vasculitis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/effect-of-avacopan-a-selective-c5a-receptor-inhibitor-on-kidney-function-in-patients-with-anca-associated-vasculitis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-avacopan-a-selective-c5a-receptor-inhibitor-on-kidney-function-in-patients-with-anca-associated-vasculitis/