Background/Purpose: Rheumatoid arthritis-associated interstitial lung disease (RA-ILD) is a prevalent and morbid condition leading to premature death in 10% of those affected. The TRAIL1 trial was a randomized, double-blinded, placebo-controlled, phase 2 study of safety, tolerability and efficacy of pirfenidone in patients with RA-ILD.
Methods: The TRAIL1 trial recruited patients aged 18 to 85 years with established RA-ILD at 33 sites in 4 countries. The primary endpoint was the incidence of the composite of decline from baseline in percent predicted forced vital capacity (FVC%) of 10% or greater or death during the 52-week treatment period. Key secondary endpoints included change in absolute and FVC% over 52 weeks.Safety was reflected by differences between the treatment arms for the rate of adverse events, serious adverse events, acute exacerbations, hospitalizations, and all-cause mortality.
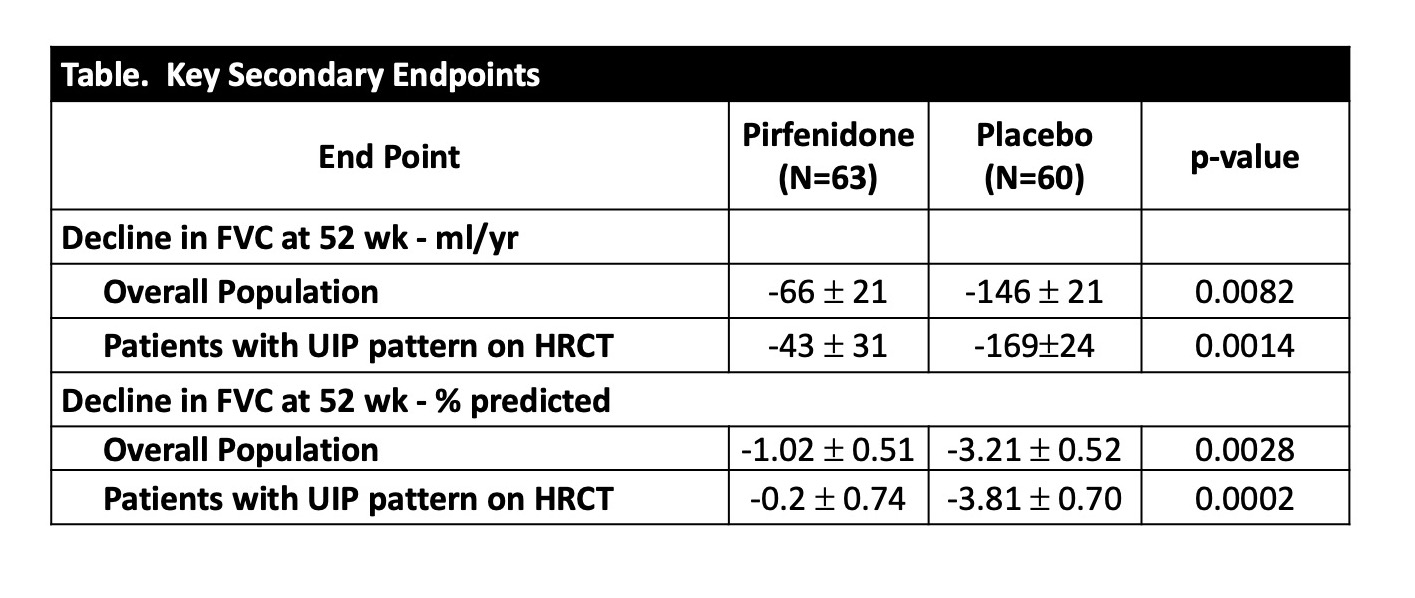
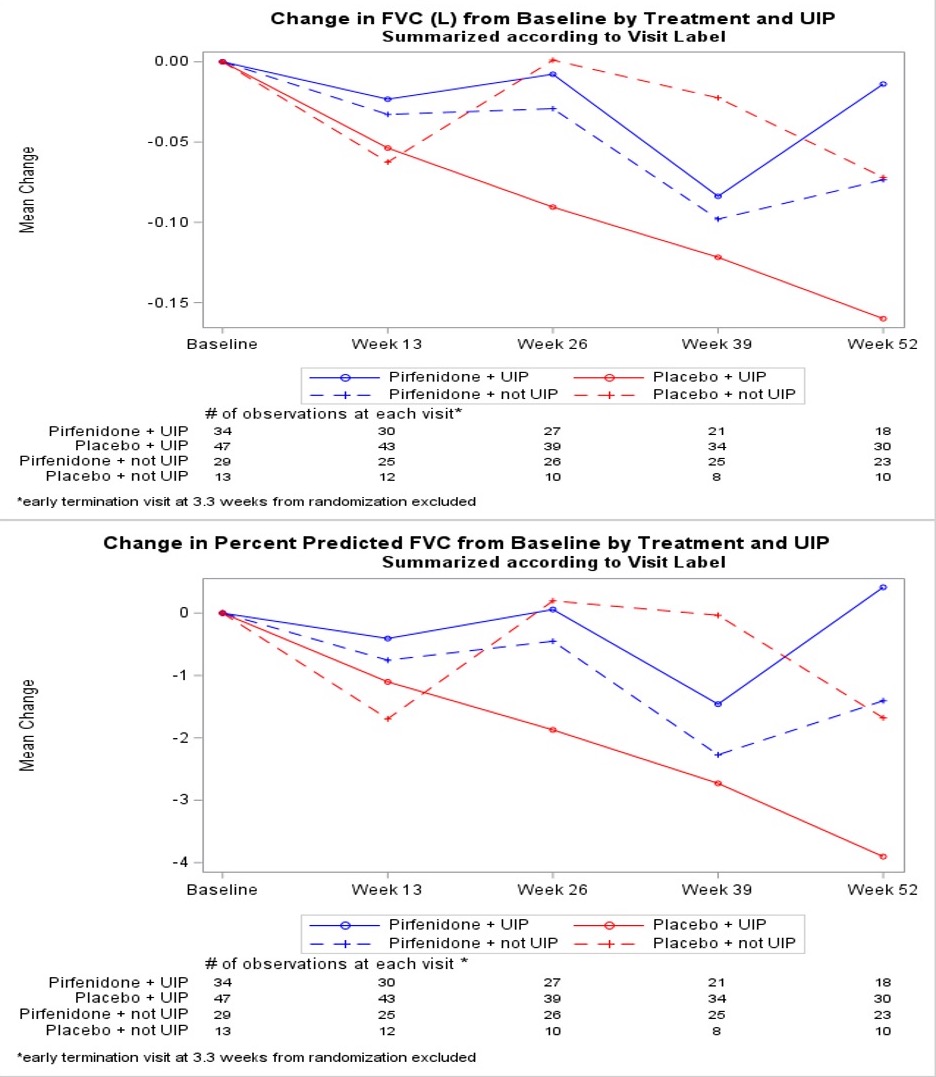
Results: With a randomization target of 270 participants, the study was stopped due to slow recruitment exacerbated by the COVID-19 pandemic. A total of 231 subjects provided consent and 123 were randomized. The proportions who met the primary endpoint were 11% on pirfenidone vs. 15% on placebo [OR=0.67 (0.22, 2.03), p=0.48]. Subjects on pirfenidone had a slower rate of decline in lung function as measured by estimated annual change in FVC(ml)(-66 vs. -146, p=0.0082) and FVC% (-1.02 vs. -3.21, p=0.0028) (Table and Figure 1). This effect on decline was also seen when analyzed within participants with baseline usual interstitial pneumonia (UIP) pattern on high resolution computed tomography (HRCT) (FVC(ml)(-43 vs. -169, p=0.0014) and FVC% (-0.2 vs. -3.81, p=0.0002) (Table and Figure 2). There was no significant difference in the rate of treatment-emergent serious adverse events.
Conclusion: Although TRAIL1 was underpowered to detect a difference in the composite primary endpoint, pirfenidone was found to be safe and slowed decline of FVC over time in subjects with RA-ILD. This effect was more pronounced in those with a UIP pattern on baseline HRCT.
To cite this abstract in AMA style:
solomon j, Woodhead F, Danoff S, Haynes-Harp S, Naik T, Spino C, Hurwitz S, Maurer R, Chambers D, Kolb M, Goldberg H, Rosas I. A Randomized, Double-Blinded, Placebo-Controlled, Phase 2 Study of Safety, Tolerability and Efficacy of Pirfenidone in Patients with Rheumatoid Arthritis Interstitial Lung Disease [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-randomized-double-blinded-placebo-controlled-phase-2-study-of-safety-tolerability-and-efficacy-of-pirfenidone-in-patients-with-rheumatoid-arthritis-interstitial-lung-disease/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-randomized-double-blinded-placebo-controlled-phase-2-study-of-safety-tolerability-and-efficacy-of-pirfenidone-in-patients-with-rheumatoid-arthritis-interstitial-lung-disease/