Session Information
Session Type: Poster Session D
Session Time: 8:30AM-10:30AM
Background/Purpose: Coronavirus Disease 2019 (COVID-19), caused by Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2), is associated with a wide range of clinical manifestations, including autoimmune features and autoantibody production. Autoantibody production has been implicated in other acute viral infections; however, the extent and breadth of autoantibodies present during acute COVID-19 has been less characterized.
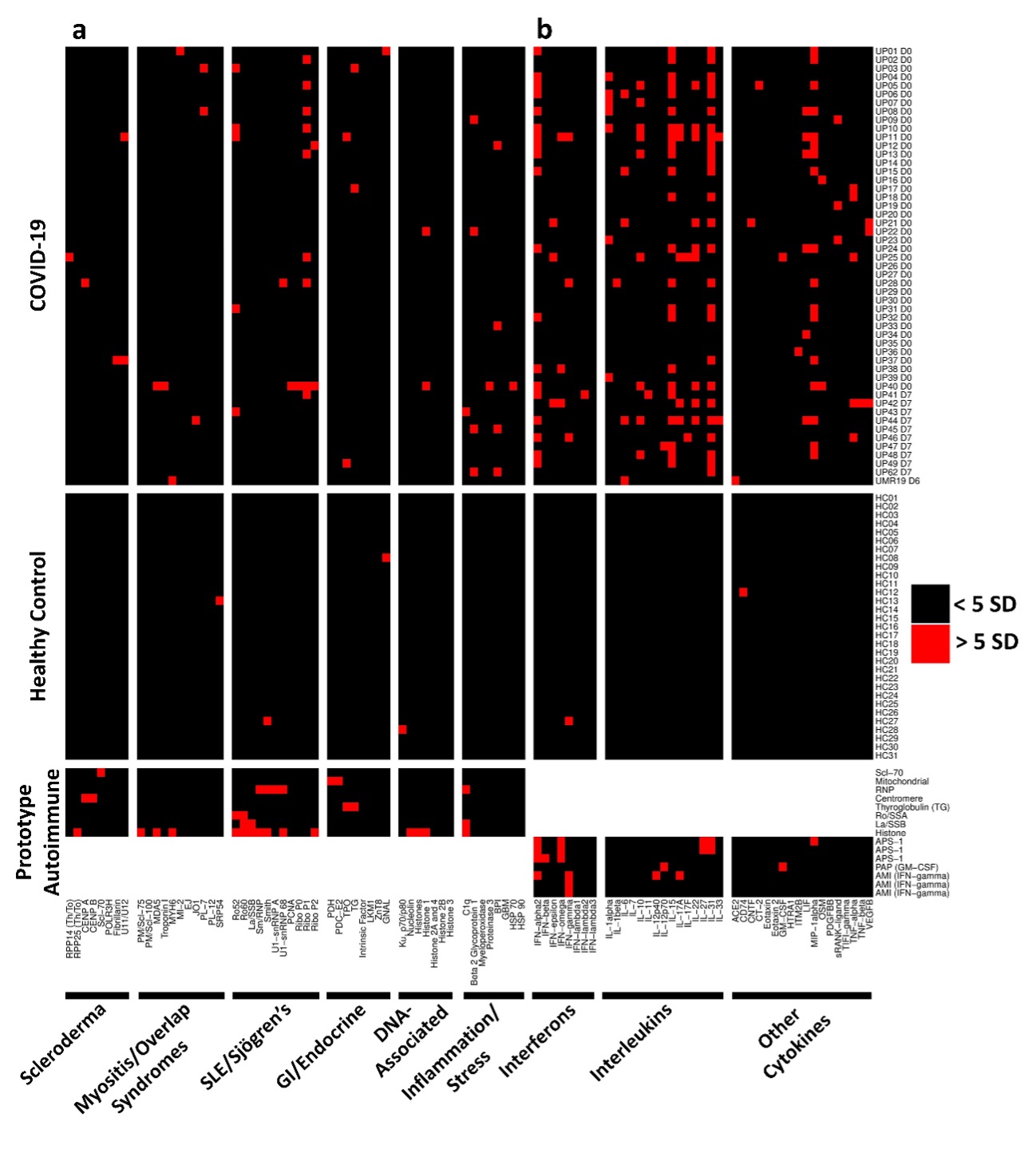
Methods: We developed three different protein arrays to measure hallmark IgG autoantibodies associated with Connective Tissue Diseases (CTDs), Anti-Cytokine Antibodies (ACA), and anti-viral antibody responses in 147 hospitalized COVID-19 patients in three different centers. The CTD autoantibody array included prominent antigens (Figure 1a, left to right) targeted in systemic sclerosis, myositis and overlap syndromes, systemic lupus erythematosus and Sjögren’s syndrome, gastrointestinal and endocrine autoimmune disorders, chromatin-associated antigens, and miscellaneous antigens, including proteins targeted in vasculitis. The ACA array included antigens represented within the interferon, interleukin or miscellaneous category (Figure 1b, left to right).
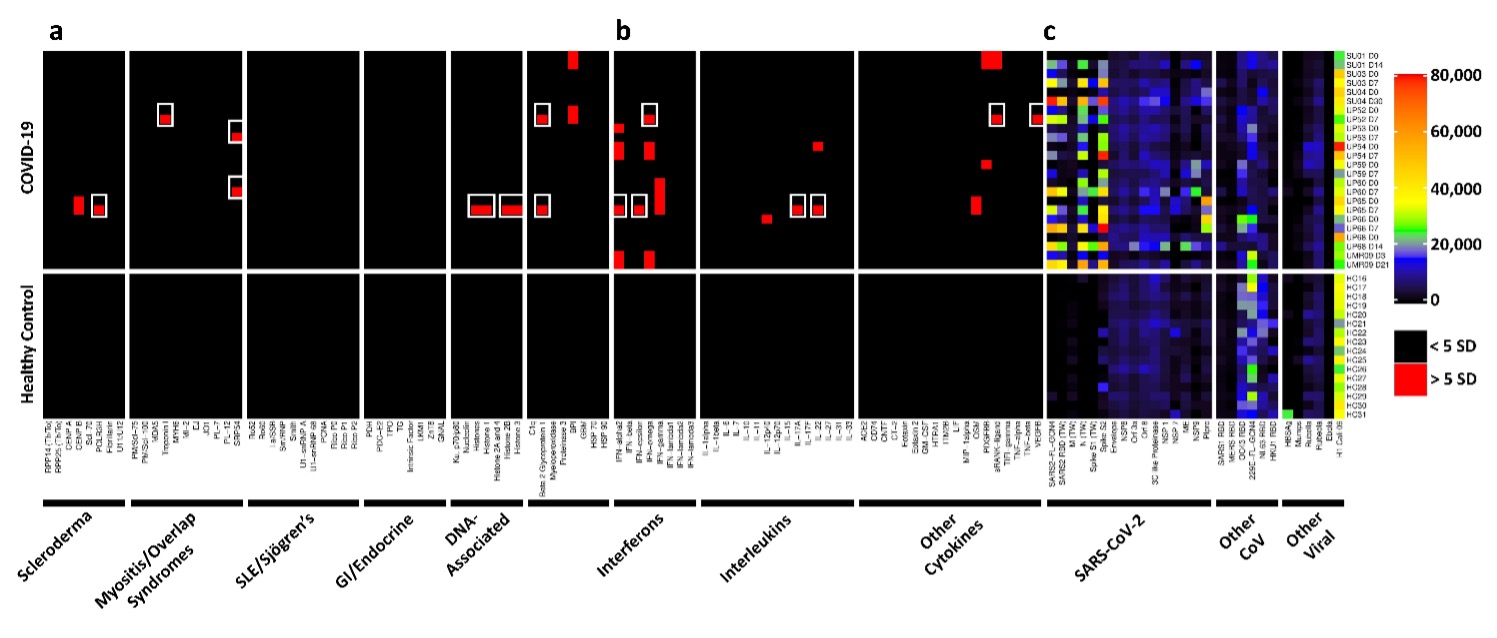
Results: Autoantibodies were identified in 49% of patients, but in < 15% of healthy controls. When present, autoantibodies largely targeted autoantigens associated with rare disorders such as myositis, systemic sclerosis and CTD overlap syndromes. Ribosomal P proteins (P0, P1, and P2) were most prominently targeted (10 of 50 patients, 20%), but were not found in any of the healthy controls. Patients with CTD autoantibodies tended to demonstrate one or a few specificities whereas ACA were more prevalent, and patients often had antibodies to multiple cytokines (Figure 1). Rare patients were identified with IgG antibodies against angiotensin converting enzyme-2 (ACE-2). Longitudinal analysis identified an expansion of autoantigen reactivity in a subset of patients at the second available time point (Figure 2). Focusing our analysis in patients early in their infection course based on spike/RBD antibody seroconversion between consecutive samples, we could identify a subset of CTD autoantibodies and ACAs that developed de novo following SARS-CoV-2 infection while others were transient (Figure 3, sequential samples in white boxes that demonstrated de novo development of CTD autoantibodies and ACAs). Autoantibodies tracked with longitudinal development of IgG antibodies that recognized SARS-CoV-2 structural proteins such as S1, S2, M, N and a subset of non-structural proteins, but not proteins from influenza, seasonal coronaviruses or other pathogenic viruses.
Conclusion: We conclude that SARS-CoV-2 infection a) is accompanied by increased prevalence of CTD-related autoantibodies and ACAs, b) in a subset of patients causes development of new-onset IgG autoantibodies, and c) that autoantibody development is positively correlated with immune responses to SARS-CoV-2 proteins.
To cite this abstract in AMA style:
Chang S, Feng A, Meng W, Apostolidis S, Mack E, Artandi M, Barman L, Bennett K, Chakraborty S, Chang I, Cheung P, Chinthrajah S, Dhingra S, Do E, Finck A, Gaano A, Gessner R, Giannini H, Gonzalez J, Greib S, Gündisch M, Hsu A, Kuo A, Manohar M, Mao R, Neeli I, Neubauer A, Oniyide O, Powell A, Puri R, Renz H, Schapiro J, Weidenbacher P, Wittman R, Ahuja N, Chung H, Jagannathan P, James J, Kim P, Meyer N, Nadeau K, Radic M, Robinson W, Singh U, Wang T, Wherry J, Skevaki C, Luning Prak E, Utz P. New-Onset IgG Autoantibodies in Hospitalized Patients with COVID-19 [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/new-onset-igg-autoantibodies-in-hospitalized-patients-with-covid-19/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/new-onset-igg-autoantibodies-in-hospitalized-patients-with-covid-19/