Session Information
Date: Sunday, November 7, 2021
Title: Plenary II (0956–0961)
Session Type: Plenary Session
Session Time: 11:30AM-11:45AM
Background/Purpose: The CARRA STOP-JIA study compared the effectiveness of the CARRA Consensus Treatment Plans (CTPs) in achieving clinical inactive disease (CID) in untreated polyarticular JIA (pJIA) at 12 months using a prospective, observational study design. The primary results were reported previously [“The CARRA STOP-JIA Study: A Comparative Effectiveness Study of CARRA Consensus Treatment Plans for Untreated Polyarticular JIA”, Kimura et al, Arthritis Rheumatol, In press]. Patients were enrolled into the CARRA Registry, which allows for long-term follow-up of this cohort. Here we report on the impact of initial CTP choices on outcomes at 24 mo.
Methods: STOP-JIA compared 3 CARRA CTPs: 1) Step Up (SU) – starting conventional synthetic disease modifying anti-rheumatic drug (csDMARD), adding biologic (b)DMARD if needed after ≥ 3 months; 2) Early Combination (EC) – csDMARD and bDMARD started together; and 3) Biologic First (BF) – starting bDMARD monotherapy, adding csDMARD if needed after ≥ 3 months. There was no randomization. Data were collected every 3 months using the CARRA Registry for the first 12 months and every 6 months thereafter. Patients with 24 months of follow-up were included in this analysis. The primary outcome was the proportion of children achieving CID off glucocorticoids (GC) at 24 months. Propensity score (PS) weighting (linear probability model) was used to balance baseline differences in potential confounders between CTPs. Secondary outcomes included the clinical Juvenile Arthritis Disease Activity Score based on 10 joints (cJADAS10), the Pediatric ACR 70 (pACR70) and patient-reported outcomes (PROs).
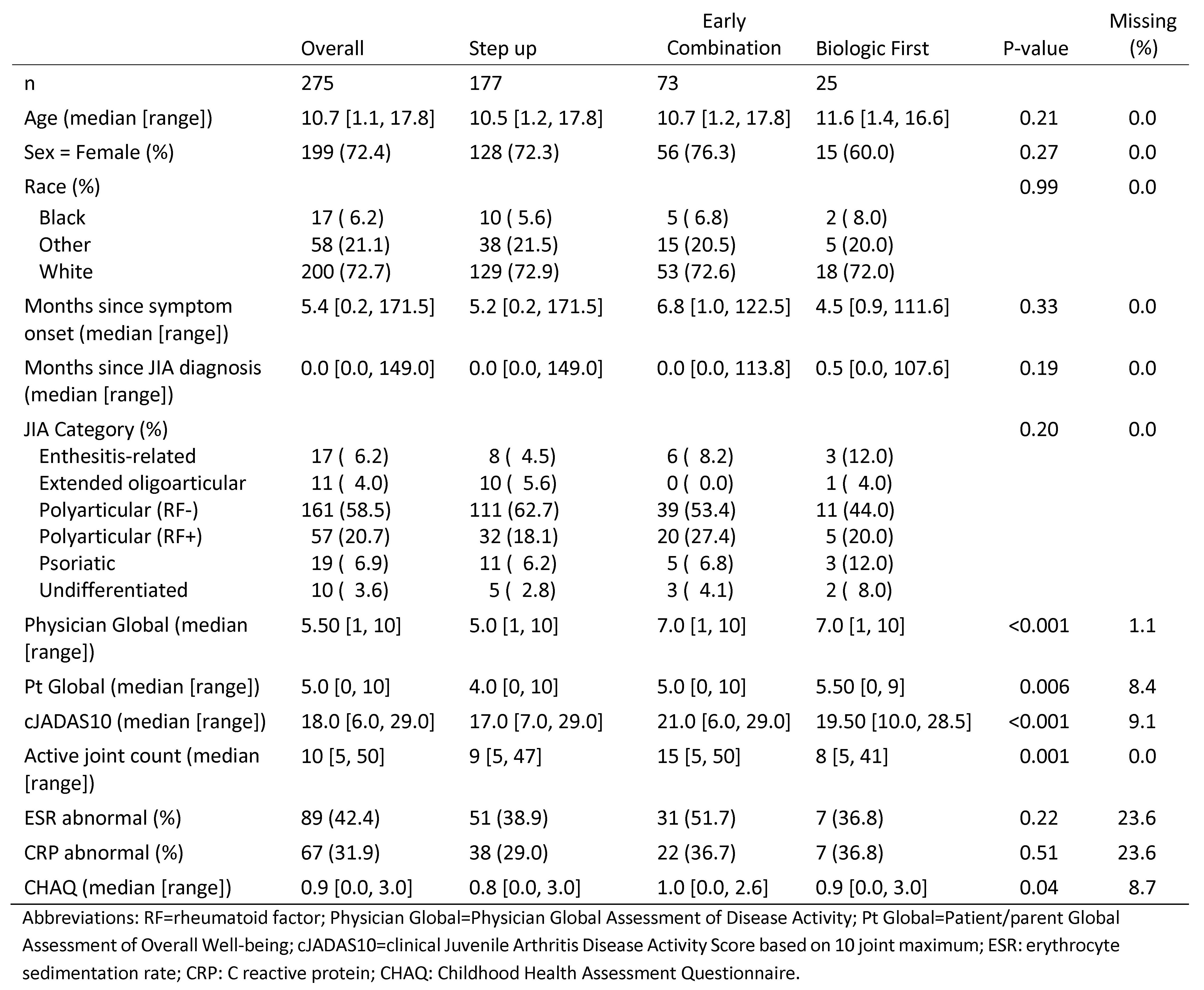
Results: 291 participants had a 24 month visit (188 Step Up, 76 Early Combination, 27 Biologic First). There were significant baseline differences between CTP groups for several variables, including JIA categories, number of active joints, Physician Global Assessment of Disease Activity, and cJADAS10 scores (Table 1). Patients in CID at 24 months was 42% for SU, 52% EC, and 44% BF (at 12 months: 32%, 37%, and 24% respectively), with a statistically significant difference (p=0.006) between the SU and EC groups after PS weighting at 24 months (Table 2). There were no significant differences between proportions of patients achieving cJADAS10 inactive disease (ID) (≤2.5) or the pACR70. All groups continued to improve at 24 months compared to 12 months. At 12 months/24 months, proportion of cJADAS10 ≤2.5 in SU: 45% vs 59%; EC: 60% vs 66%; BF: 48% vs. 57%. At 12 months/24 months, proportion of pACR70 in SU: 47% vs 74%; EC: 59% vs 83%; BF: 44% vs 74%. Figure 1A shows the proportions of each group’s JADAS activity over the 24 months. All groups also showed improvement in PROMIS® pain interference or mobility measures from baseline (Figure 1B). Seventeen SAEs were reported, most commonly infections.
Conclusion: While there was a significant difference in CID favoring Early Combination versus Step Up at 24 months, there were no significant differences between CTPs for other study outcomes. All groups did continue to improve in cJADAS10 and PRO measures. Additional analyses will include comparison of remission on medications (CID for 12 months) and patients ever achieving CID between CTPs.
To cite this abstract in AMA style:
Kimura Y, Ringold S, Tomlinson G, Schanberg L, Dennos A, Riordan M, Del Gaizo V, Murphy K, Weiss P, Feldman B, Natter M, CARRA Registry Investigators T. The Childhood Arthritis and Rheumatology Research Alliance Start Time Optimization of Biologic Therapy in Polyarticular JIA (STOP-JIA) Study: 24-Month Outcomes [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/the-childhood-arthritis-and-rheumatology-research-alliance-start-time-optimization-of-biologic-therapy-in-polyarticular-jia-stop-jia-study-24-month-outcomes/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-childhood-arthritis-and-rheumatology-research-alliance-start-time-optimization-of-biologic-therapy-in-polyarticular-jia-stop-jia-study-24-month-outcomes/