Session Information
Date: Sunday, November 7, 2021
Title: Patient Outcomes, Preferences, & Attitudes Poster II: Measurements (0739–0763)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: A significant heterogeneity exists across outcome domains and outcome instruments measuring the effect of self-management interventions targeting people with inflammatory arthritis (IA). Thus, it is currently difficult to perform evidence synthesis assessing the effect of self-management interventions, and variability in the quality of the applied outcome measurement instruments exists. The objective of this study was to identify outcome measure instruments applied to measure the effect of self-management interventions targeting patients with IA.
Methods: Measures of self-management in IA were identified through an informative systematic literature review as described by OMERACT (1) and the Core Outcome Measures in Effectiveness Trials (COMET) (2) which focus on mapping outcome domains from earlier studies to inform the development of a COS. Trials were eligible for inclusion if the intervention was described as “self-management intervention“, and the study population comprised of people with any IA: RA, PsA or SpA. Two authors independently assessed trials for eligibility and extracted information on study characteristics, outcome domains, and outcome measurement instruments reported. With discrepancies, discussion among all authors was conducted. Outcome domains and the outcome measurement instruments were sorted and categorized into domains and sub-domains using the OMERACT Framework based on 3 core areas: (i) Death, (ii) Life Impact, and (iii) Pathophysiological manifestations, plus resource use/economical evaluation which is highly recommended, but not defined as a mandatory core area.
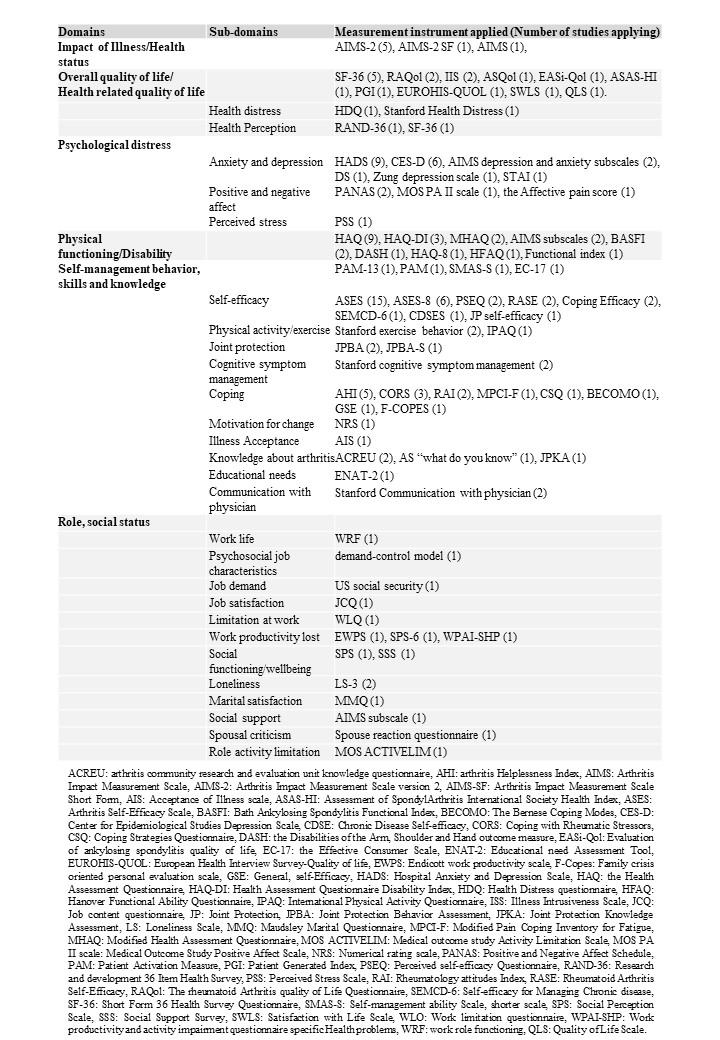
Results: From a total of 2502 records, we included 38 trials, published between 1988 and 2021. The studies most often based their self-management intervention on cognitive behavioral therapy and/or self-efficacy theory focusing on education of arthritis, medication, lifestyle, and self-management skill training involving problem-solving and goal setting. Across the trials, we identified 12 different outcome domains and 47 sub domains, comprising 128 different measurement instruments. The outcome domains and outcome sub-domains are illustrated in Figure 1. The identified outcome domains were primarily within the ‘Life impact’ core area. Self-efficacy and health-related quality of life was the most commonly applied domains in 30 and 16 studies, respectively. The domains and measurement instrument within the ‘Life impact’ core area are listed in Table 1.
Conclusion: The outcome domains and measurement instruments reported in self-management interventions including IA population, were widely diverse. This study provides a foundation for the development of a core outcome set for use in future trials.
Registration
The protocol was registered in PROSPERO (ID CRD42021238749).
1. OMERACT Handbook 2019. Available at: https://www.dropbox.com/s/fd3673fsma45qe0/OMERACT Handbook Chapter 4 Apr 16 2019.pdf?dl=0
2. Williamson PR, Altman DG, et al. The COMET Handbook: Version 1.0, 2017. Available at: https://trialsjournal.biomedcentral.com/articles/10.1186/s13063-017-1978-4
To cite this abstract in AMA style:
Hansen C, Esbensen B, Christensen R, De Thurah A, Cromhout P. Outcome Reporting in Self-management Interventions for Inflammatory Arthritis Trials: A Systematic Review of Outcome Measures Covering Self-management Domains [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/outcome-reporting-in-self-management-interventions-for-inflammatory-arthritis-trials-a-systematic-review-of-outcome-measures-covering-self-management-domains/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/outcome-reporting-in-self-management-interventions-for-inflammatory-arthritis-trials-a-systematic-review-of-outcome-measures-covering-self-management-domains/