Session Information
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
Background/Purpose: Whether the likelihood of a cancer diagnosis in idiopathic inflammatory myopathy (IIM) patients differs by autoantibody type is not fully characterized. To inform cancer surveillance in IIM patients, in a large, tertiary referral IIM cohort, we determined whether cancer occurrence differs by autoantibody type from that expected based on the general US population.
Methods: Patients enrolled in our myositis cohort was retrospectively reviewed from 2007-2020 for adult patients who met one of these criteria: (i) Probable or definite DM/PM by Peter and Bohan, (ii) IMNM by the 2003 ENMC Criteria, (iii) Classic DM rash (Gottron’s/heliotrope) and consistent histopathology on skin biopsy. Myositis specific and associated autoantibodies were determined systematically in our research lab using Euroimmun line blot (16 Ag IgG), ELISA (Mi2 and TIF1ƴ by MBL, HMGCR by INOVA Diagnostics), and in-house immunoprecipitation (NXP2). Three time windows were analyzed: cohort enrollment onward, IIM symptom-onset onward, and within 3 years of IIM symptom onset (-3 to +3 years). The Surveillance, Epidemiology, and End Results (SEER) registry was used to compare the observed number of cancers to the expected number of cancers in the general population, adjusted for calendar year, age, sex, and race. Standardized prevalence ratios of cancer (SPR) – the observed/expected prevalence of cancer over an interval of time – were determined.
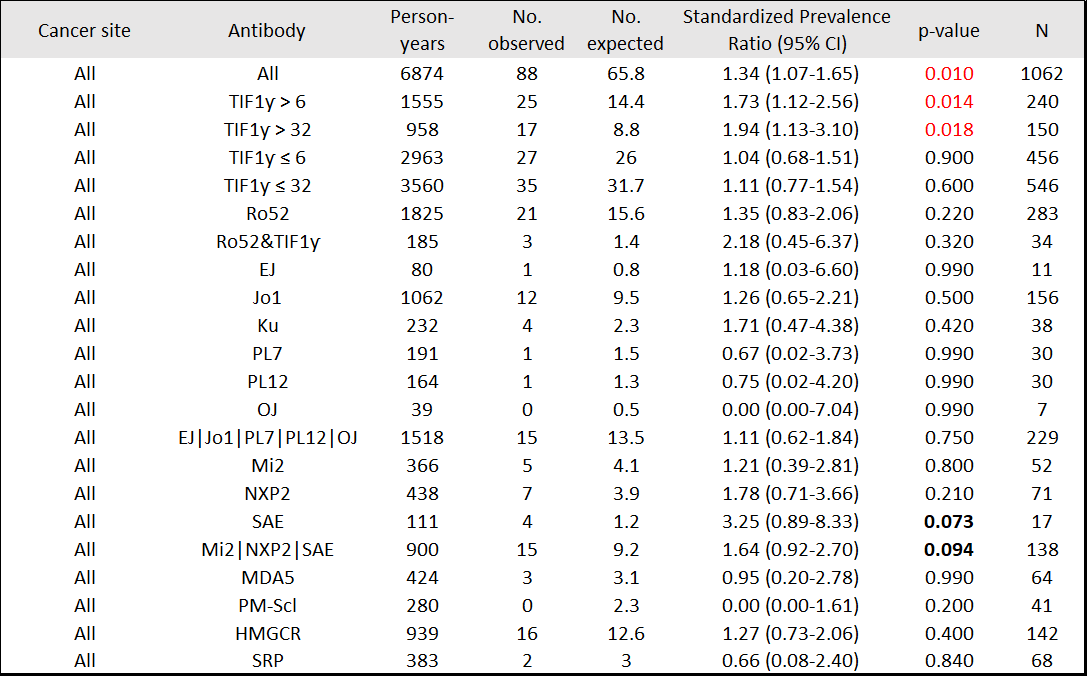
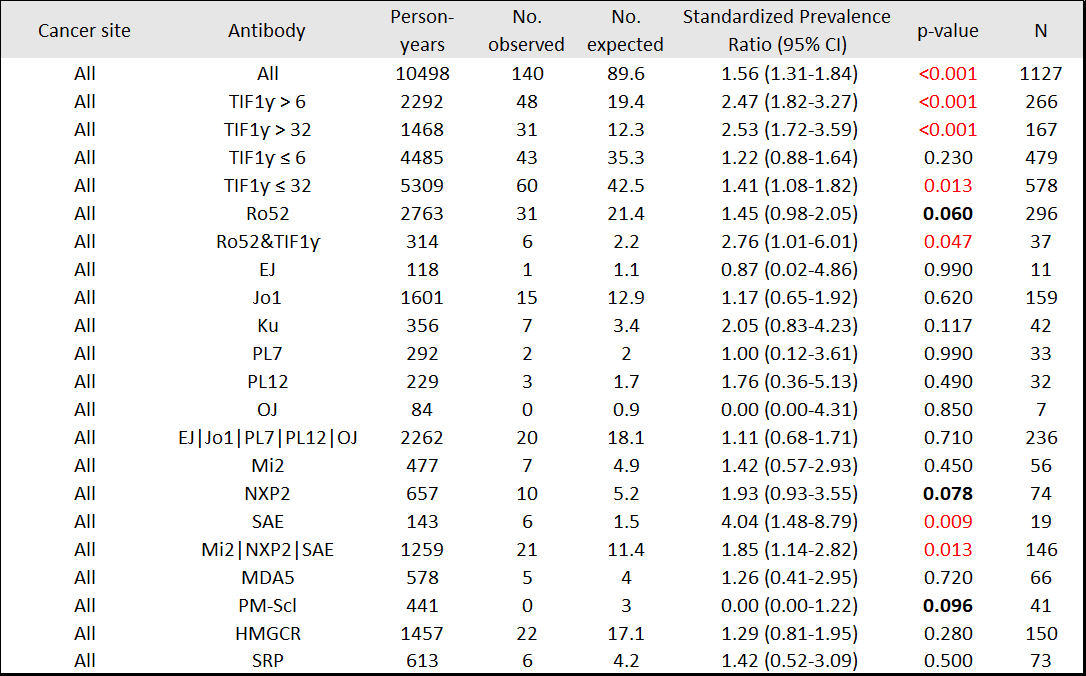
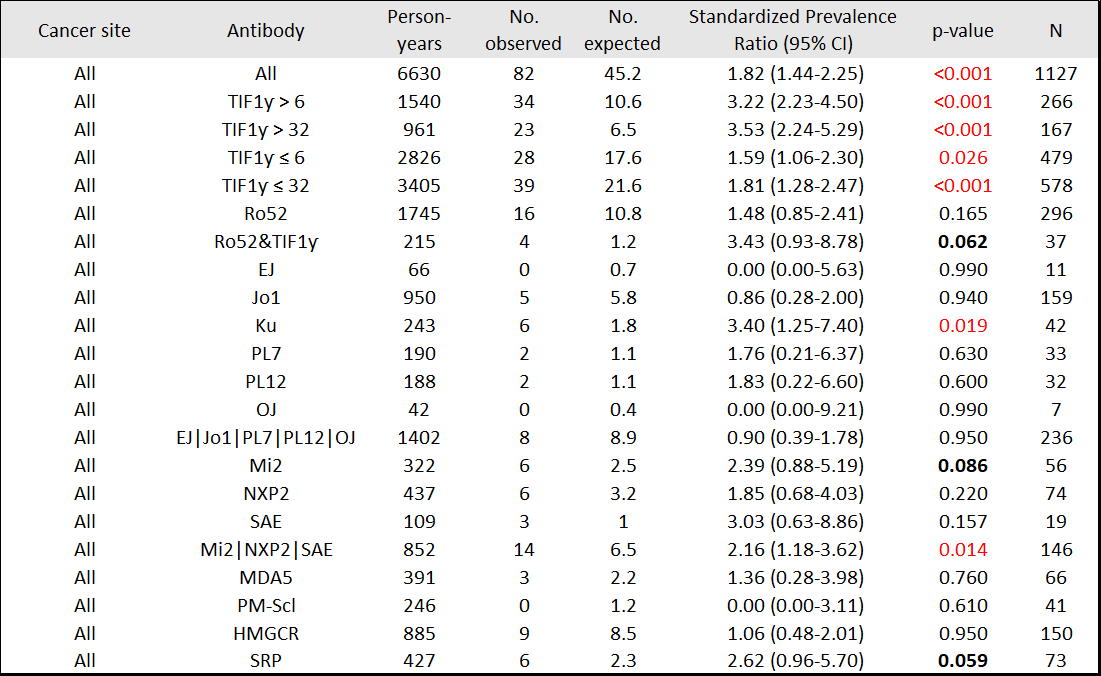
Results: 1174 patients, including 201 (17%) with a history of cancer, were studied. Median (IQR) time from IIM symptom onset to enrollment was 1.68 (0.75-3.8) yrs. From enrollment onward (5.21 [2.64-8.64] yrs; 89 diagnosed with cancer), patients with TIF1ƴ autoantibodies ( >6 units) had higher than expected cancer period prevalence (SPR=1.73, 95%CI 1.12-2.56), whereas patients negative for anti-TIF1ƴ did not (SPR=1.04, 95%CI 0.68-1.51) [Table 1]. From IIM symptom onset onward (7.95 [4.92-11.86] yrs; 131 diagnosed with cancer, 25.2% within 0-1 yr, 28.2% >1-3 yrs, 14.5% >3-5 yrs, 32.1% >5 yrs), patients with anti-TIF1ƴ had higher than expected period prevalence of cancer, mainly breast (SPR=2.95, 95%CI 1.69-4.70) and ovarian (SPR=16.63, 95%CI 6.10-36.19). Patients with anti-SAE also had higher than expected cancer period prevalence (SPR=4.04, 95%CI 1.48-8.79). Patients with anti-Mi2 had higher than expected period prevalence of gastrointestinal cancers (SPR=6.03, 95%CI 1.24-17.61) [Table 2]. Within 3 yrs of IIM symptom onset (93 diagnosed with cancer), patients with anti-TIF1ƴ had higher than expected period prevalence of breast and ovarian cancer [Table 3]. Patients with DM autoantibodies (Mi2, SAE, or NXP2) had higher than expected cancer period prevalence.
Conclusion: In this tertiary referral center cohort, anti-TIF1ƴ was associated with higher occurrence of breast and ovarian cancers. Other DM-specific autoantibodies (Mi2, SAE, or NXP2) were associated with higher cancer occurrence. No cancer association (positive or negative) was observed for antisynthetase antibodies. These data suggest that intensive cancer surveillance is not needed in all IIM patients, and that autoantibody type may inform cancer occurrence, site and timing. Funded in part by NIAMS K23AR075898.
To cite this abstract in AMA style:
Mecoli C, Igusa T, Chen M, Wang X, Albayda J, Paik J, Tiniakou E, Adler B, Richardson C, Kelly W, Danoff S, Mammen A, Platz E, Rosen A, Christopher-Stine L, Casciola-Rosen L, Shah A. Standardized Prevalence Ratios of Cancer in Idiopathic Inflammatory Myositis [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/standardized-prevalence-ratios-of-cancer-in-idiopathic-inflammatory-myositis/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/standardized-prevalence-ratios-of-cancer-in-idiopathic-inflammatory-myositis/