Session Information
Date: Sunday, November 7, 2021
Title: Epidemiology & Public Health Poster II: Inflammatory Arthritis – RA, SpA, & Gout (0560–0593)
Session Type: Poster Session B
Session Time: 8:30AM-10:30AM
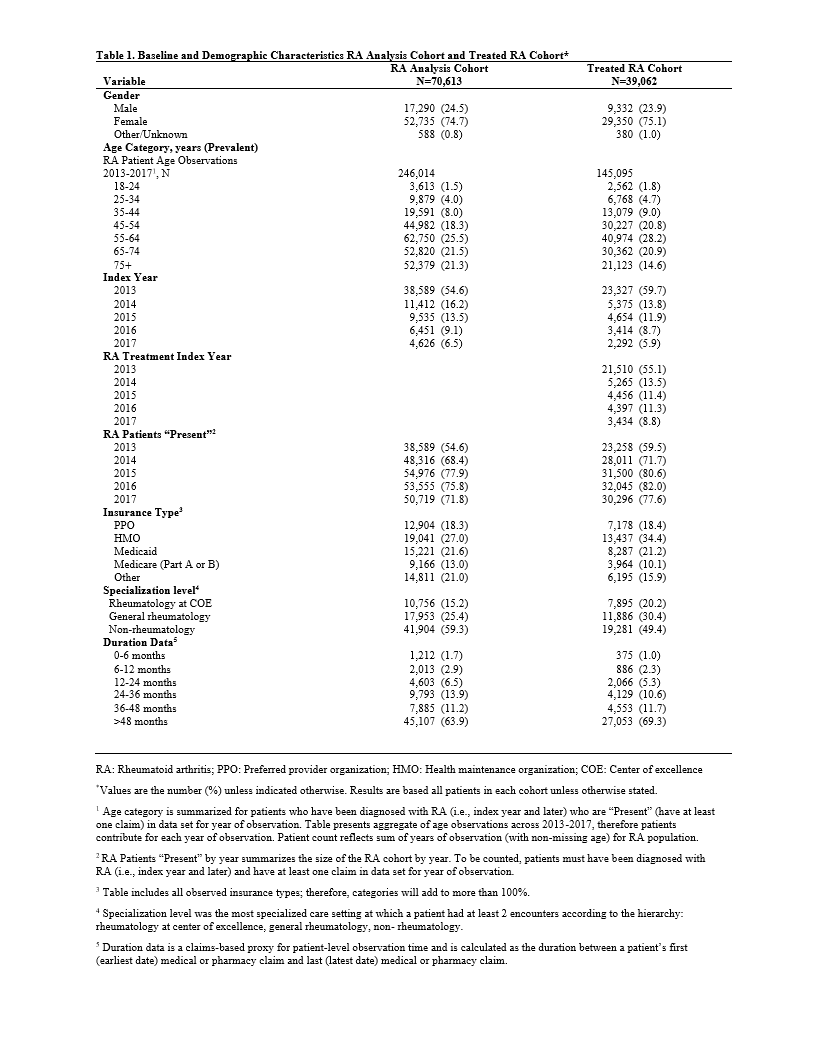
Background/Purpose: A real-world current state of RA patients in Massachusetts (MA) is analyzed to provide a novel assessment of demographics, treatment patterns, and clinical settings of care, with particular focus on initial and subsequent prescribing patterns of DMARDs.
Methods: This was a retrospective cohort analysis of the MA Center for Health Information and Analysis all-payers claims database from 2013-2017, which aggregates claims data covering ~70% of the population, excluding traditional (fee-for-service) Medicare. The analytic cohort included all adults (18+ years) with ³2 RA-related claims on different dates. The treated cohort required ³1 claim for a conventional synthetic, biologic, or targeted synthetic DMARD (csDMARD, bDMARD, or tsDMARD, respectively). Further analysis included treatment patterns by year, demographics, medication, and specialization level (non-rheumatology (i.e. PCP and other specialty), general rheumatology, or a designated RA Center of Excellence).
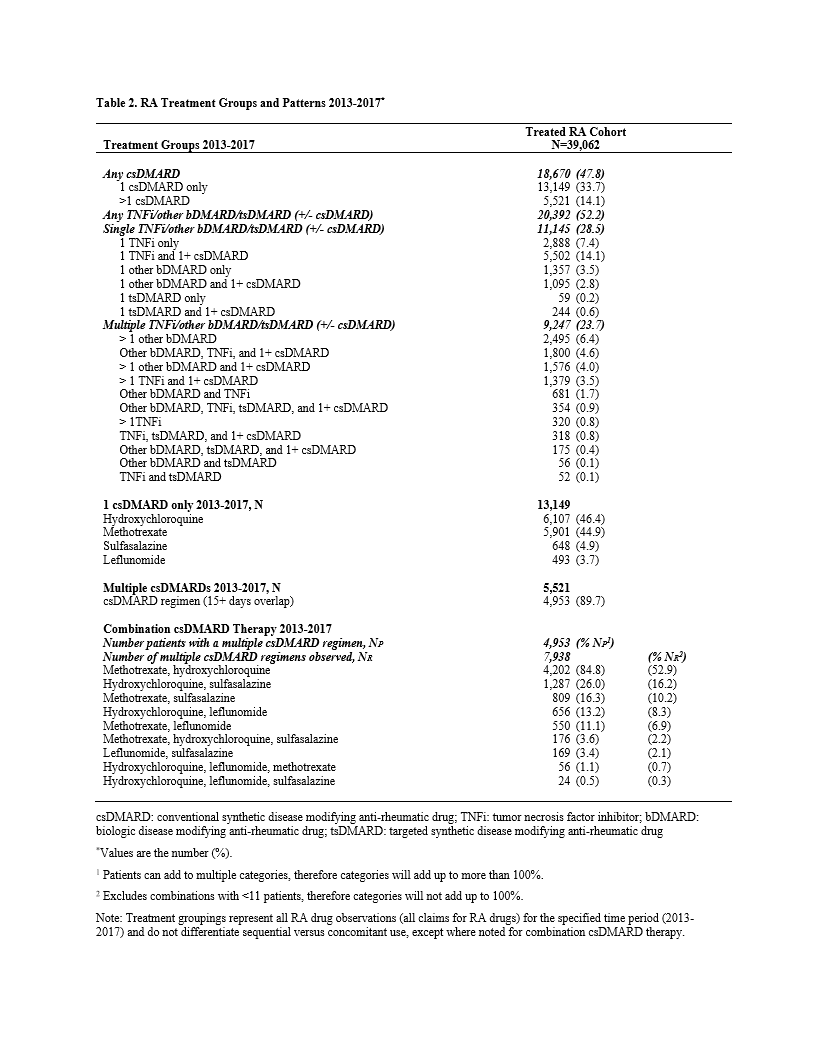
Results: The analytic cohort comprised 70,613 patients. The majority were female (74.7%), age ³45 years (86.6%), index date in 2013 (54.6%), the latter suggesting established RA diagnoses. (Table 1) The treated cohort included 39,062 patients (55.3%), of which 13,149 (33.7%) received only a single csDMARD, predominately HCQ (46.4%) or MTX (44.9%). HCQ as a single agent was more common in female patients of childbearing age, with HCQ use falling steadily with increasing age, opposite that of MTX. An additional 14.1% received >1 csDMARD, either sequentially (1.4%) or in combination (12.7%). MTX plus HCQ was the most common regimen in 84.8% of patients on combination therapy. Triple therapy (MTX, HCQ, and SSZ) was taken by 3.6% of patients receiving combination csDMARD-only therapy. (Table 2)
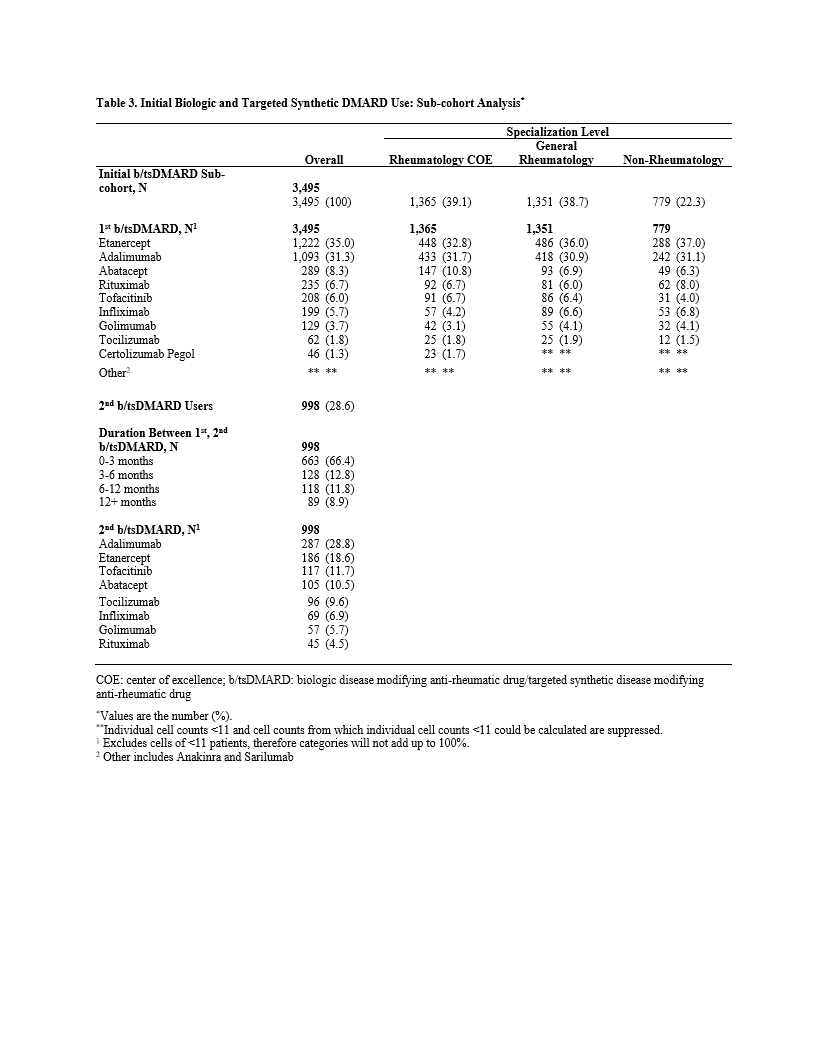
A bDMARD and/or tsDMARD was taken by 20,392 patients (52.2%), of which 9,247 (45.3%) had claims for >1 bDMARD or tsDMARD, suggesting a regimen change. (Table 2) Among a subcohort of 3,495 patients who took ³1 bDMARD or tsDMARD with ³6 months of prescribing data prior to the first prescription, etanercept (ETN) and adalimumab (ADA) were the most common initial agents (66.3%). Of those, 998 patients (28.6%) had claims for a second bDMARD or tsDMARD. Of 998, a TNF-inhibitor (TNFi) was the first agent in 818 (82.0%), and the second agent for the majority was another TNFi (70.3%), followed by other bDMARD (20.5%) and tsDMARD (9.2%). (Table 3)
Specialization level was rheumatology for 40.7% of the analysis cohort, 50.6% of the treated cohort, and 77.7% of those initiating a bDMARD or tsDMARD. (Table 1). Drug selection varied by specialization level. Among the 8,390 (21.5%) patients receiving a single TNFi agent (+/- csDMARD), more patients received ETN and ADA in rheumatology settings and infliximab in non-rheumatology settings.
Conclusion: Overall, DMARD prescribing was low, and there was a high level of HCQ monotherapy and TNFi use. An unexpectedly high number of RA patients in MA were treated in non-rheumatology settings. This first state-wide current state analysis of RA in MA elucidates treatment patterns for RA in the United States, highlighting opportunities for practice improvement and helping to direct treatments in a timely and patient-centered fashion.
To cite this abstract in AMA style:
Matza M, Fox D, Larholt K, Fritsche D, Apgar E, Puthran M, Hirsch G, Bolster M. Rheumatoid Arthritis Treatment Patterns in Massachusetts: Informative Findings from Insurance Claims Data [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/rheumatoid-arthritis-treatment-patterns-in-massachusetts-informative-findings-from-insurance-claims-data/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rheumatoid-arthritis-treatment-patterns-in-massachusetts-informative-findings-from-insurance-claims-data/