Session Information
Date: Saturday, November 6, 2021
Title: SLE – Diagnosis, Manifestations, & Outcomes Poster I: Diagnosis (0323–0356)
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: The multianalyte assay panel (MAP) consists of cell-bound complement activation products (CB-CAPs) with lupus and non-lupus autoantibodies combined in an algorithm (Dervieux et al., J Immunol Methods 2017). The test is intended for patients suspected of systemic lupus erythematosus (SLE) to aid the diagnosis. We demonstrated previously that the MAP has clinical utility, as it facilitates SLE diagnosis and treatment decisions (Wallace et al., Lupus Sci & Med 2019). As the number of patients with a positive MAP in that study was small, the present study was conducted to enrich the population of the MAP positive patients and to generate additional data on the clinical utility of MAP.
Methods: Systematic multicenter retrospective review of medical charts was conducted at 12 rheumatology practices in the United States. Adult patients for chart review were selected by Exagen based on the MAP score. To decrease the risk of bias, sets of 5 possible eligible patients were identified for each site. Each set included 2 negative (< -0.1) and 3 positive ( > 0.1): tier-1 positive [MAP(Tier1+)]; tier-2 score > 1 [MAP(High+)]; tier-2 > 0.1 and ≤ 1 [MAP(Low+)]). The cutoff of 1 was based on the likelihood ratio positive of the test. Charts were reviewed at T0 (when the MAP was ordered), T1 (when the results were reviewed) and, if available, T2 (latest visit ≥8 months after T1). Statistical analysis (R) consisted of Kaplan-Meier survival analysis with Cox proportional hazard model and ordered logistic regression, as appropriate.
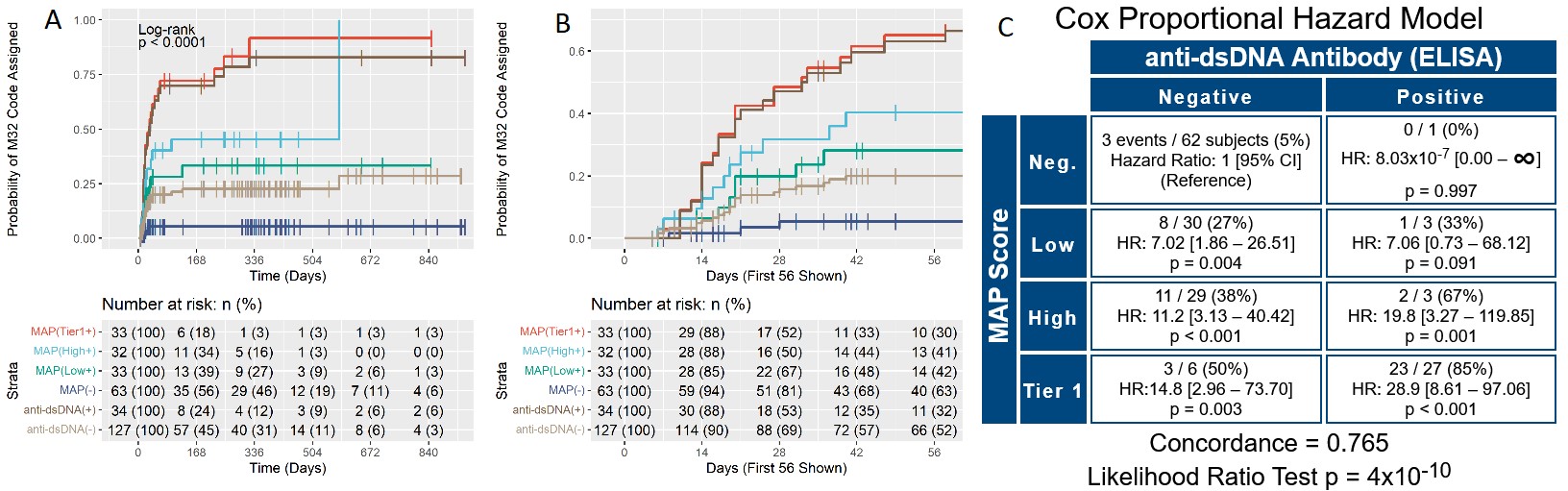
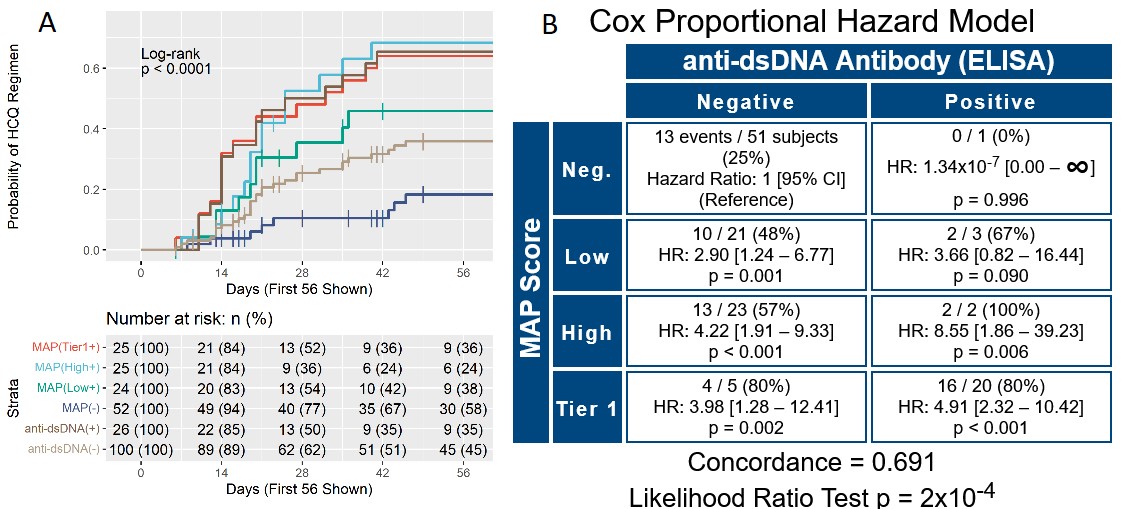
Results: T0 and T1 were performed for 161 patients. All sites but one reviewed charts as multiples of 5 (5 – 25 patients per site). Charts were reviewed also at T2 for a subset of 90 patients (56%). At T0, physician confidence in SLE diagnosis was low for 93 (58%), moderate for 49 (30%), and high for 19 (12%) patients. Odds of higher confidence in SLE diagnosis increased during the study by 1.74-fold for every unit of increase of the MAP score (p< 0.001). The lupus ICD-10 section (M32) was used as a proxy for diagnosis. Positive MAP led to increased assignment of an SLE code by Cox proportional hazard model (Figure 1). In particular, M32 was assigned to 22 of 65 (34%) MAP positive patients who were anti-dsDNA negative. In addition, MAP negative was superior to anti-dsDNA negative at excluding of an SLE code, as more anti-dsDNA negative than MAP negative patients were at risk of M32 assignment (63% vs 52%) (Figure 1B). Only 3 of the 63 MAP negative patients (5%) were assigned a lupus ICD-10 code during the study, indicating a 95% probability of excluding the SLE diagnosis in MAP negative patients. We also evaluated the use of hydroxychloroquine (HCQ) during the study in the 126 patients who were prescribed HCQ after T0. Kaplan-Meier curves and hazard ratios for initiation of HCQ show that MAP positivity led to HCQ treatment, and that MAP negative was superior to anti-dsDNA negative in avoiding HCQ prescription (58% vs. 45%) (Figure 2).
Conclusion: This study demonstrates that the MAP helps to both diagnose and exclude SLE in patients suspected of the disease and, importantly, informs appropriate treatment decisions in this patient population.
Panels A and B. Kaplan-Meier time-to-event curves for assignment of the M32 ICD_10 section over time. Curves show the percent probability of being assigned the M32 section after T0 for the 4 study groups : tier_1 (MAP(Tier1+)), high tier_2 (MAP(High+)), low tier_2 (MAP(Low+)) negative (MAP(-)), in addition to anti-dsDNA positive (anti-dsDNA(+)) and negative (anti-dsDNA(-)) patients throughout the study. The X-axis reports the number of days since T0. Panels A and B report the same data analysis, with panel B allowing better visualization of the initial portion of the survival curves. Panel C. Cox proportional hazard model comparing the MAP test score (Neg.: negative; Low: low tier_2; High: high tier_2, Tier 1: tier_1 positive) vs. anti-dsDNA antibodies for assignment of the M32 section. In each quadrant, the numerators represent the number of subjects that developed an M32 section after T0 (events, n=51 in total) while the denominators represent all subjects in that quadrant. Concordance and p value of the likelihood ratio test are also reported. For data analysis, we used the date of the visit when the M32 section was recorded in the ICD_10 list.
Panel A. Kaplan-Meier time-to-event curves for use of HCQ over time. Curves show the percent probability of using HCQ after T0 for the 4 study groups: tier_1 (MAP(Tier1+)), high tier_2 (MAP(High+)), low tier_2 (MAP(Low+)) negative (MAP(-)), in addition to anti-dsDNA positive (anti-dsDNA(+)) and negative (anti-dsDNA(-)) patients throughout the study. The X-axis reports the number of days since T0 and was truncated at 56 days to allow better visualization of the initial portion of the survival curves. Panel B. Cox proportional hazard model comparing the MAP score (Neg.: negative; Low: low tier_2; High: high tier_2, Tier 1: tier_1 positive) vs. anti-dsDNA antibodies for use of HCQ. In each quadrant, the numerators represent the number of subjects on HCQ after T0 (events, n=60 in total) while the denominators represent all subjects in that quadrant. Concordance and p value of the likelihood ratio test are also reported. For data analysis, we used the date of the visit when HCQ use was recorded in the medication list.
To cite this abstract in AMA style:
Alexander R, Rey S, Conklin J, Domingues V, Ahmed M, Qureshi J, Weinstein A. A Multianalyte Assay Panel with Cell-bound Complement Activation Products Demonstrates Clinical Utility for the Diagnosis and Treatment of Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/a-multianalyte-assay-panel-with-cell-bound-complement-activation-products-demonstrates-clinical-utility-for-the-diagnosis-and-treatment-of-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-multianalyte-assay-panel-with-cell-bound-complement-activation-products-demonstrates-clinical-utility-for-the-diagnosis-and-treatment-of-systemic-lupus-erythematosus/