Session Information
Session Type: Poster Session A
Session Time: 8:30AM-10:30AM
Background/Purpose: Hypoxia and subsequent oxidative stress are early events in the RA joint and contribute to the activation of synovial fibroblasts (SF). Small molecule inhibitors targeting the members of the bromodomain and extra-terminal (BET) protein family (BRD2, BRD3, BRD4) have anti-inflammatory properties in RA. Here we analyzed the potential of BET inhibitors in regulating stress response pathways in RA SF.
Methods: SF were obtained from shoulder and hand joints of RA patients undergoing joint replacement surgery. We mimicked hypoxic conditions by treatment of SF with dimethyloxalylglycine (DMOG; 0.5 mM, 24 h). Stabilization of HIF1α and GLUT1 protein expression were assessed by Western blotting. Oxidative stress was induced by treatment of SF with 4-hydroxynonenal (4-HNE; 5 µM, 48h), a lipid peroxidation product present in synovial fluids, and TNF (10 ng/ml, 48h). Experiments were performed in absence and presence of the pan-BET inhibitor I-BET151 (1 µM). The expression of hypoxic response (VEGFA, PDK1, GLUT1, HK2) and oxidative stress response genes (CAT, GPX1, SOD2, HMOX1) was measured by Real-time PCR (n=7-10). Autophagy was assessed by Western blotting using LC3B-II as an autophagy marker (n=7).
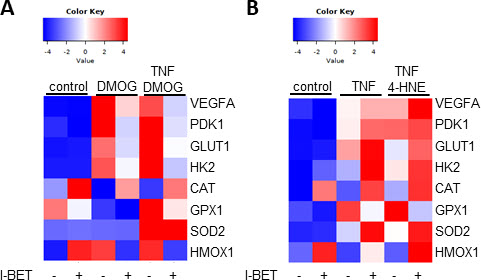
Results: Treatment of SF with DMOG stabilized HIF1α protein. I-BET treatment suppressed the DMOG-induced expression of the hypoxic response genes VEGFA, PDK1, GLUT1 and HK2 (Figure 1A). Hypoxic conditions induced the GLUT1 protein expression by 101.8-fold (± 108.2-fold), which was suppressed by 62.2% (± 32.1%) in presence of I-BET (p< 0.05). TNF treatment induced the expression of all measured stress response genes. Oxidative stress further induced the TNF-induced expression of VEGFA, PDK1, GLUT1, HK2 and CAT (Figure 1B). In contrast to results obtained under hypoxic conditions, I-BET treatment further increased the expression of hypoxia response genes under oxidative stress conditions. We have detected similarities and differences regarding the BET-meditated regulation of oxidative stress response genes under different stress conditions. I-BET increased basal HMOX1 and CAT expression in all conditions, and suppressed GPX1 expression under hypoxia and oxidative stress. In contrast, BET inhibition decreased the expression of SOD2 only during oxidative stress but not under hypoxia. I-BET further increased the expression of HMOX1 during oxidative stress, but suppressed the expression of HMOX1 during hypoxia. I-BET induced the accumulation of LC3B-II protein by 2.7 (± 1.4)-fold, which was further increased to 4.5 (± 2.9)-fold in presence of DMOG (p< 0.05). Oxidative stress conditions did not further induce the I-BET-induced expression of LC3B-II.
Conclusion: BET protein inhibition affects stress-response target genes in a stimulus-dependent manner. Our data suggest that hypoxia and oxidative stress conditions regulate individual stress response genes via distinct enhancers. Furthermore, we have identified BET proteins as regulators of autophagy.
To cite this abstract in AMA style:
Seifritz T, Züllig T, Moser L, Distler O, Ospelt C, Klein K. Bromodomain Protein-regulated Stress Response in Rheumatoid Arthritis Synovial Fibroblasts [abstract]. Arthritis Rheumatol. 2021; 73 (suppl 9). https://acrabstracts.org/abstract/bromodomain-protein-regulated-stress-response-in-rheumatoid-arthritis-synovial-fibroblasts/. Accessed .« Back to ACR Convergence 2021
ACR Meeting Abstracts - https://acrabstracts.org/abstract/bromodomain-protein-regulated-stress-response-in-rheumatoid-arthritis-synovial-fibroblasts/