Session Information
Date: Monday, November 9, 2020
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Clinical trials in early diffuse systemic sclerosis (SSc) using the modified Rodnan skin score (mRSS) as the primary outcome have been largely negative. This may reflect limitations of clinical trial design or outcome measures, as opposed to therapeutic failures. Our objective was to assess if stratification could enhance early diffuse SSc clinical trial design and analysis. Our primary hypothesis was that given the predominance of RNA polymerase III (RNAP3) in recent clinical trials and its association with severe skin involvement, stratification by RNAP3 or other factors could improve trial design.
Methods: We used a large, observational Scleroderma Center cohort from a US tertiary care referral site. We identified early diffuse SSc patients first seen between 1980 and 2015. We included those with < 36 months from first non-Raynaud manifestation and ≥3 recorded mRSS scores in the analysis. Our modeling strategy reflected typical disease duration inclusion criteria for ongoing clinical trials: < 18 months and < 36 months of disease. We used descriptive, time to event, group-based trajectory modeling to determine mRSS trajectory groups, multivariable regression modeling as well as classification tree analyses (CTA) to assess contributing factors to predict mRSS trajectory groupings. We analyzed the following variables: age, gender, race, antibody, skin thickness progression rate (STPR), tendon friction rubs (TFR), elevated ESR and medication use.
Results: We found 514 patients who met criteria with < 36 months of disease, of which 403 had < 18 months of disease at first visit (Table 1). The median disease duration was 0.90 (IQR 0.59, 1.39) years at first visit, median follow-up 10.2 (4.5, 16.3) years with 14 (8, 23) SSc clinic visits. The majority, 273 (53%) were RNAP3 positive, and 137 (27%) were Scl-70 positive.
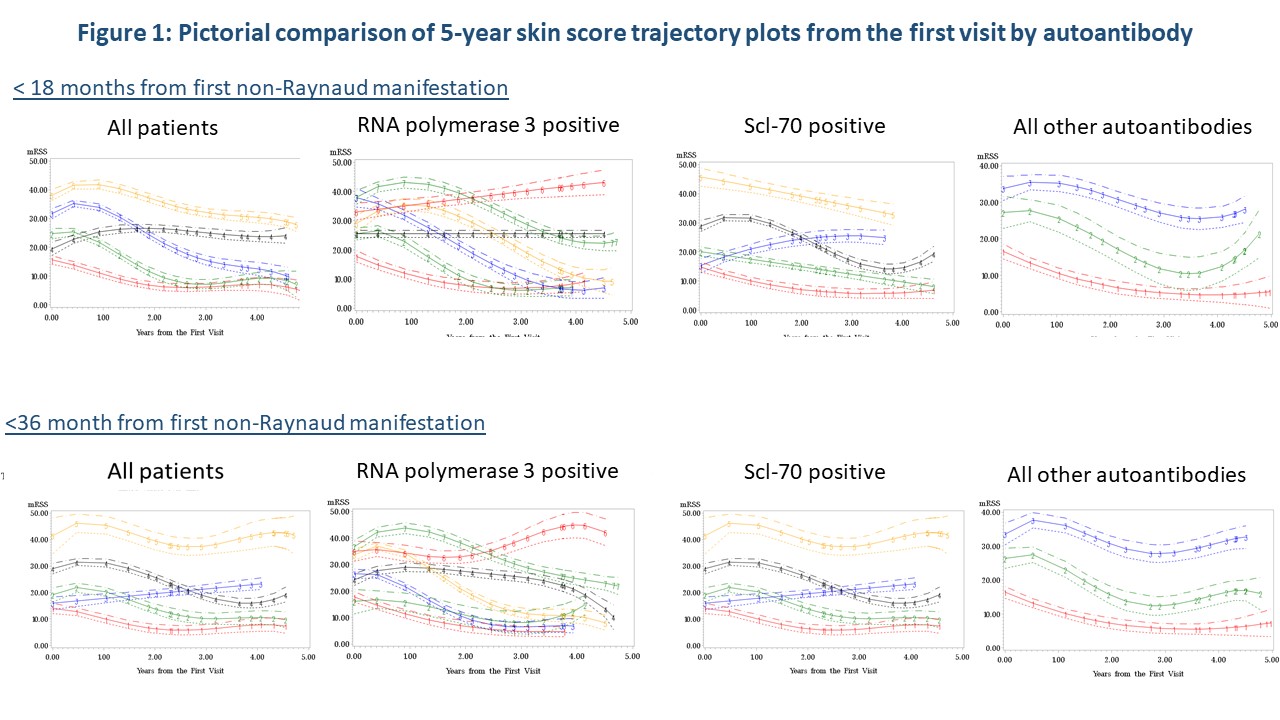
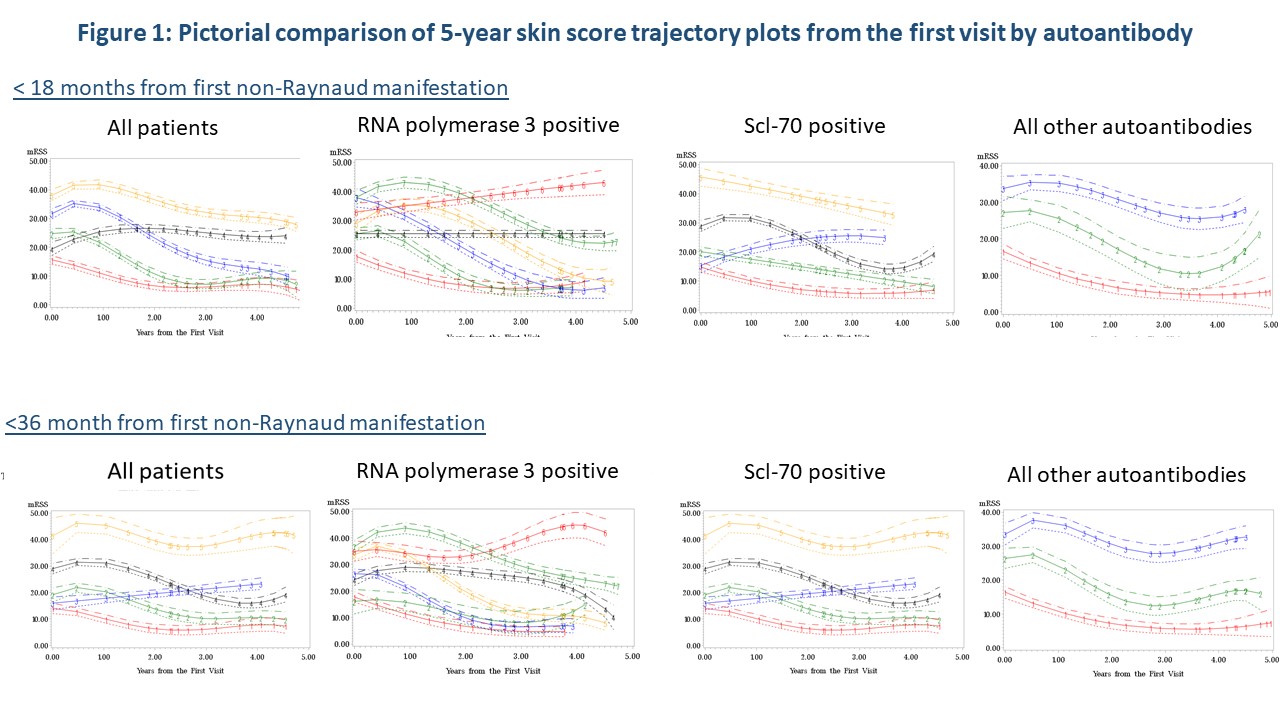
Initial time-to event analysis demonstrated a significant difference between time to peak mRSS for those positive for Scl-70 (p=0.04) or RNA3 positive (p=0.02). Trajectory modeling showed distinctly different mRSS trajectory patterns over 5 years by autoantibody regardless of disease duration (Figure 1). Regression analysis confirmed that STPR (p< 0.0001), TFR (p=0.002) and RNAP3 (p=0.01) were predictors of trajectory groupings. CTA analysis (Figure 3) demonstrated that measured characteristics performed better in predicting trajectory grouping for < 18 months than predicting trajectory groups for < 36 months of disease. STPR and presence of TFR were suggested as predictive factors for trajectory grouping for both < 18 months and < 36 months disease duration.
Conclusion: STPR, presence of TFR and RNAP3 antibody are consistently associated with mRSS trajectory patterns over time using multiple analysis methods. STPR and TFR are early nodal breaks in CTA analysis, suggesting they may be preferred stratification strategies over RNAP3 status. As STPR is intended to be used at the initial SSc clinic visit, TFR presence is the more feasible data-supported stratification strategy for early diffuse clinical trials.
 Table 1: First Visit Demographic and Systemic Sclerosis Charcteristics
Table 1: First Visit Demographic and Systemic Sclerosis Charcteristics
To cite this abstract in AMA style:
Domsic R, Gao S, Laffoon M, Wisniewski S, Lafyatis R, Steen V, Medsger T. Exploring Stratification Strategies for Early Diffuse Systemic Sclerosis Clinical Trial Design [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/exploring-stratification-strategies-for-early-diffuse-systemic-sclerosis-clinical-trial-design/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/exploring-stratification-strategies-for-early-diffuse-systemic-sclerosis-clinical-trial-design/