Session Information
Session Type: Poster Session D
Session Time: 9:00AM-11:00AM
Background/Purpose: Subcutaneous (SC) tanezumab, a monoclonal antibody that inhibits nerve growth factor, was investigated for the relief of signs and symptoms of moderate-severe OA in patients (pts) for whom use of other analgesics was ineffective or not appropriate in a randomized NSAID-controlled global long-term safety study (NCT02528188). The current analysis examines the effect of tanezumab versus NSAID on clinically important improvements in pts with OA.
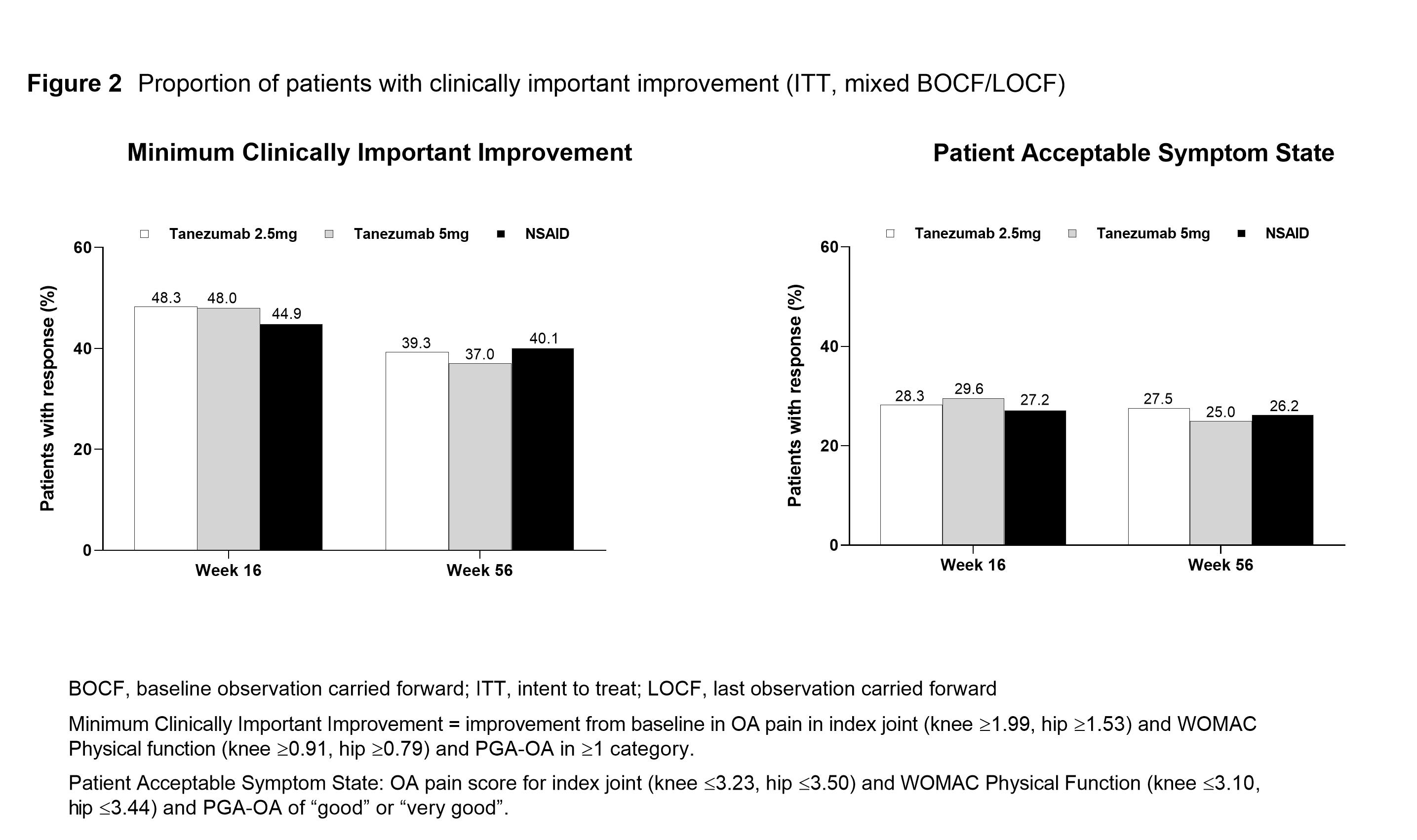
Methods: Eligible pts had hip or knee OA based on ACR criteria with x-ray confirmation; baseline (BL) WOMAC Pain and Physical Function subscale scores of ≥5; BL Pt Global Assessment of OA (PGA-OA) of “fair”, “poor”, or “very poor”; history of inadequate pain relief with acetaminophen; inadequate pain relief with/intolerance to tramadol or opioids, or unwilling to take opioids. Pts were on a qualifying, stable dose of NSAID before study entry. Pts received SC tanezumab (2.5 mg or 5 mg every 8 Wks) or oral NSAID (BID) over the 56-Wk treatment period. At Wk 16, pts who did not have the required efficacy response (≥15% reduction from BL in WOMAC Pain subscale score at Wk 2, 4, or 8, and ≥30% reduction from BL at Wk 16) were discontinued from study treatment and entered the safety follow-up period. The proportion of pts who had a reduction from BL of ≥30%, ≥50%, ≥70% or ≥90% in average weekly pain (electronic pain diary) was evaluated at Wks 16 and 56. Pt Acceptable Symptom State (PASS; the value beyond which pts consider themselves well) and Minimum Clinically Important Improvement (MCII; smallest change in measurement that signifies an important improvement in a pt’s symptoms) were evaluated at Wks 16 and 56 using Tubach and colleagues’ composite endpoints of improvement based on average pain, WOMAC Physical Function and PGA-OA (definitions provided in Figure 2).1,2 Responders in average pain, PASS, and MCII are post-hoc analyses. Unadjusted p-values are presented.
Results: In total, 3,021 pts were randomized and 2,996 received at least 1 dose of blinded SC study drug and are included in the efficacy analyses. A total of 1,312 pts completed the treatment period (42–45% across treatment groups). BL pt characteristics were similar across treatment groups: mean age 60.3–61.2 years; female 63.6–66.5%; duration of index joint OA 7.9–8.1 years; index joint was knee 84.9–85.5%, and hip in 14.5–15.1%. The proportion of pts with reductions from BL in average pain was significantly greater for tanezumab (2.5 mg, ≥30% responders; 5 mg, ≥30%, ≥50%, and ≥90% responders) versus NSAID at Wk 16 and largely similar across treatment groups at Wk 56 for all categories of response (Figure 1). There were no significant differences in the proportion of pts achieving MCII and PASS between treatment groups at Wks 16 and 56 (Figure 2).
Conclusion: The majority of tanezumab treated pts had a clinically important pain improvement (≥30%) at Wk 16, significantly more than with NSAID. The composite pain and function improvement and acceptable severity level after treatment was similar for tanezumab and NSAID.
Disclosures: Sponsored by Pfizer and Eli Lilly & Company.
References:
- Tubach F, et al. Ann Rheum Dis. 2005; 64(1): 34–7.
- Tubach F, et al. Ann Rheum Dis. 2005; 64(1): 29–33.
To cite this abstract in AMA style:
Hunter D, Neogi T, Churchill M, Shirinsky I, Omata M, White A, Guermazi A, Fountaine R, Pixton G, Viktrup L, Brown M, West C, Verburg K. Clinically Important Improvements in Patients with Osteoarthritis Treated with Subcutaneous Tanezumab: Results from a 56-Week Randomized NSAID-Controlled Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/clinically-important-improvements-in-patients-with-osteoarthritis-treated-with-subcutaneous-tanezumab-results-from-a-56-week-randomized-nsaid-controlled-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinically-important-improvements-in-patients-with-osteoarthritis-treated-with-subcutaneous-tanezumab-results-from-a-56-week-randomized-nsaid-controlled-study/