Session Information
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patients with systemic sclerosis (SSc) may be at increased risk for severe outcomes with COVID-19, given the high rate of immunosuppressive medication use, underlying lung pathologies, and accompanying co-morbid conditions. Limited data are available on outcomes of COVID-19 infection in these patients.1,2 The American College of Rheumatology (ACR) and the European League Against Rheumatism (EULAR) are collecting provider-entered COVID-19 cases in a registry of patients with rheumatic diseases.3,4 We report number and outcomes of COVID-19 cases in regularly monitored diffuse cutaneous(dc) SSc patients followed in 2 open-label extension (OLE) studies of lenabasum, an orally-administered, non-immunosuppressive, selective cannabinoid receptor type 2 (CB2) agonist.
Methods: This cohort included participants satisfying ACR/EULAR criteria for SSc who were receiving lenabasum 20 mg BID in the OLE studies in a Phase 2 (NCT02465437) or Phase 3 trial (NCT03398837). Background therapy with immunosuppressive medications was allowed. COVID-19 infection rate was discerned by review of adverse events (AEs) and required a positive COVID-19 test. Outcome of COVID-19 was provided by treating physicians.
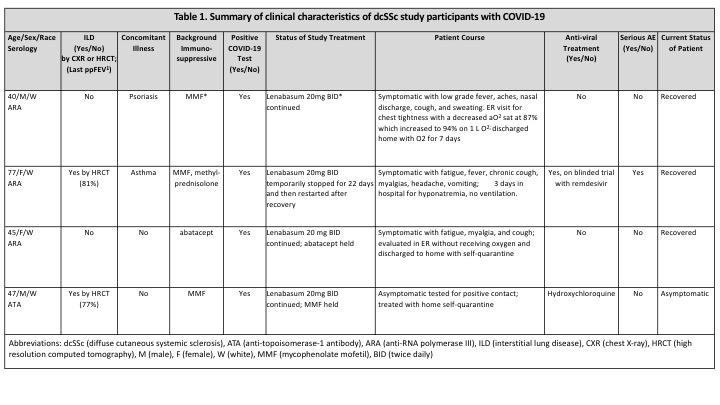
Results: Four/385 (0.11%) participants suffered a COVID-19 adverse event (AEs), including one serious AE (SAE). Mean age was 52 years (40-77), with 2 females, and all participants were white. Two had interstitial lung disease (ILD) and were being treated with mycophenolate mofetil (Table 1). One with ILD was asymptomatic and tested for Covid-19 because of contact with known COVID-19 positive individuals. This individual was treated with 5 days of hydroxychloroquine. During COVID-19 infection, 3 continued and 1 temporarily stopped lenabasum treatment due to hospitalization (SAE). This participant with ILD was hospitalized for 3 days and discharged home after 2 doses of experimental remdesivir, with full recovery 4 weeks after symptoms began. One received oxygen therapy in the emergency room (ER), then discharged home with oxygen for a week with subsequent full recovery. One symptomatic had an ER visit without requiring oxygen therapy and another asymptomatic did not have an ER visit; both did not require hospitalization and were self-quarantined.
Conclusion: The rate of confirmed COVID-19 infections (0.11%) in this cohort of dcSSc patients is about the same as the rate estimated from CDC data of June 5, 2020, adjusted for age (~0.1%). This suggests the diffuse cutaneous SSc subjects in this cohort are not more vulnerable to COVID-19 infection than the general population. All 4 trial participants despite 2 with confirmed ILD had acceptable outcomes, in the context of continued or resumed lenabasum treatments.
- Ann Rheum Dis. 2020;79(5):668‐669.
- Avouac J, Airó P, Carlier N, et al. Ann Rheum Dis. 2020 Epub ahead of print.
- ACR COVID-19 Global Rheumatology Alliance Registry https://rheum-covid.org/
- EULAR COVID-19 Database https://www.eular.org/eular_covid19_database.cfm
To cite this abstract in AMA style:
Domsic R, Chung L, Molitor J, Spiera R, Bloom B, White B, Dinh Q. Clinical Outcomes Among Participants with Diffuse Systemic Sclerosis Contracting COVID-19 During Clinical Studies of Lenabasum: A Case Series [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/clinical-outcomes-among-participants-with-diffuse-systemic-sclerosis-contracting-covid-19-during-clinical-studies-of-lenabasum-a-case-series/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/clinical-outcomes-among-participants-with-diffuse-systemic-sclerosis-contracting-covid-19-during-clinical-studies-of-lenabasum-a-case-series/