Session Information
Date: Sunday, November 8, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment Poster III
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Patient-reported outcomes (PROs) are important when evaluating treatment benefits in PsA. We present an analysis of PRO data from the SELECT-PsA 1 study.
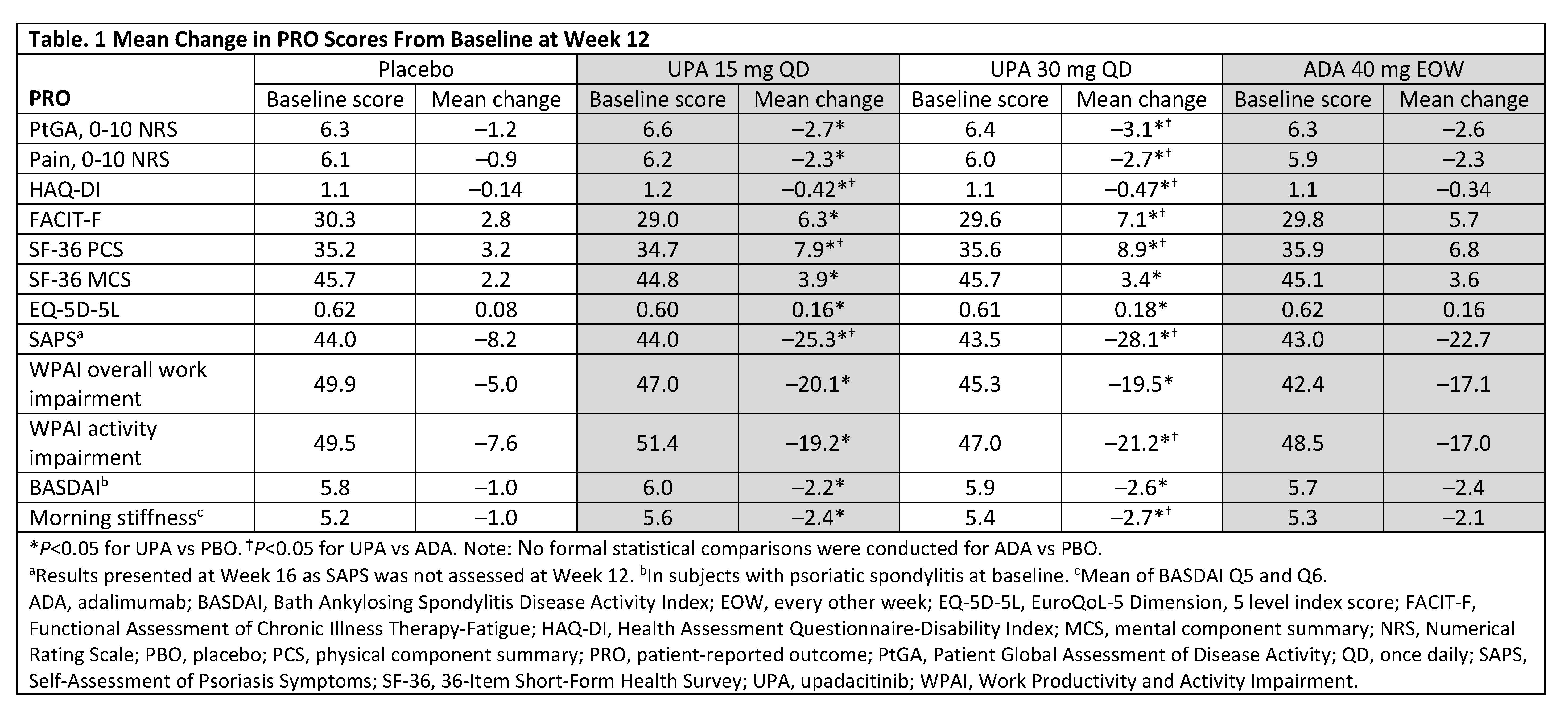
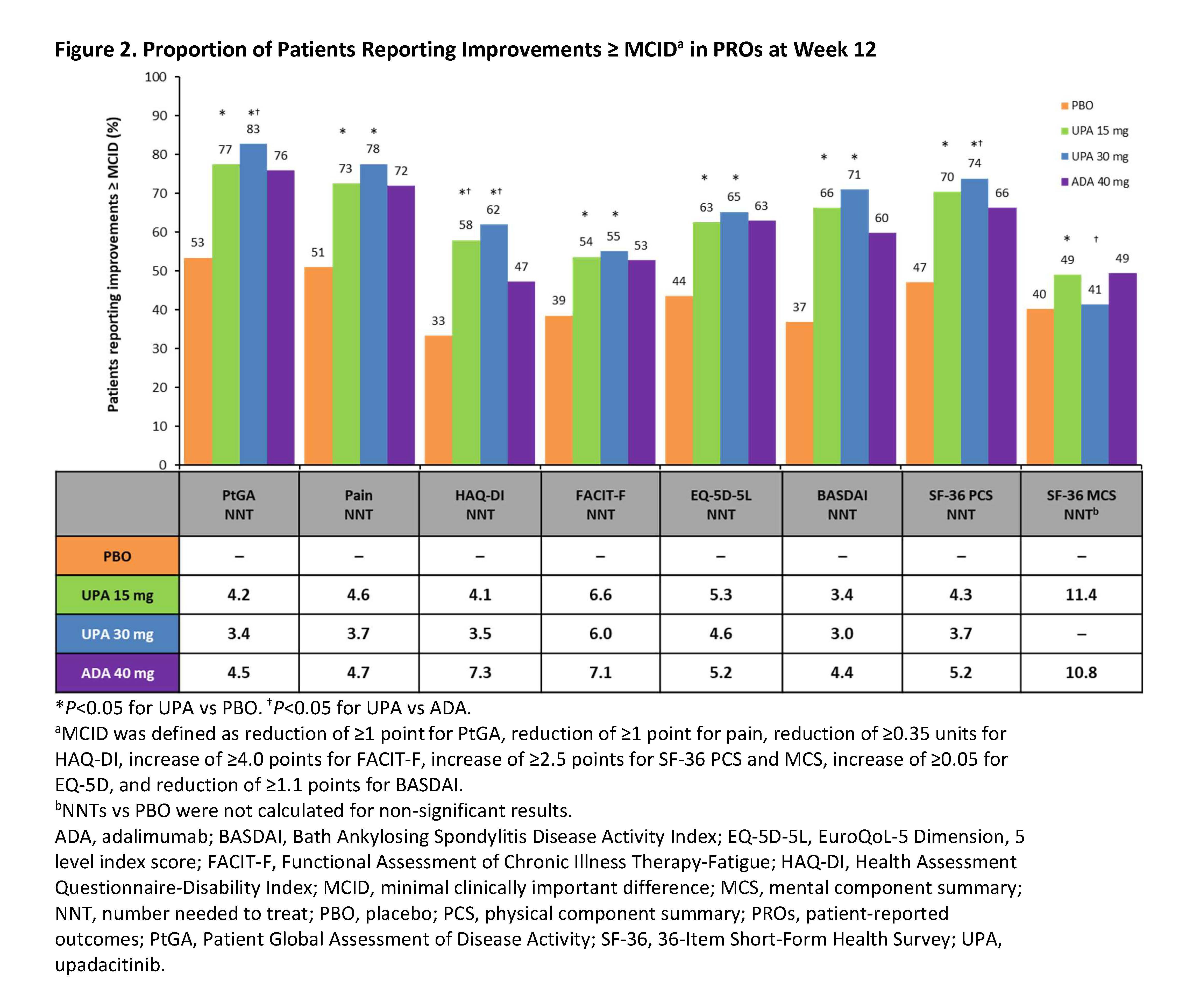
Methods: SELECT-PsA 1 (NCT03104400) is a Phase 3, randomized, placebo- (PBO) and active-controlled trial in patients with active PsA and inadequate responses to ≥1 non-biologic DMARD. Eligible patients were randomized to receive upadacitinib (UPA) 15 mg once daily (QD), UPA 30 mg QD, adalimumab (ADA) 40 mg every other week, or PBO for 24 weeks, with the primary endpoint assessment at Week 12. PROs included: Patient Global Assessment of Disease Activity (PtGA), Patient’s Assessment of Pain, HAQ-Disability Index (HAQ-DI), Functional Assessment of Chronic Illness Therapy-Fatigue, 36-Item Short-Form Health Survey (SF-36), EuroQoL-5 Dimension, 5 level, Self-Assessment of Psoriasis Symptoms (SAPS), Work Productivity and Activity Impairment, Bath Ankylosing Spondylitis Disease Activity Index (BASDAI), and morning stiffness (items 5 and 6 from the BASDAI). BASDAI was assessed in patients with presence of psoriatic spondylitis at baseline (BL). Least squares mean changes from BL to Week 12 (Week 16 for SAPS) were assessed. Percentages of patients reporting improvements ≥ minimal clinically important differences (MCID) from BL through Week 24 were compared between treatment groups. Number needed to treat (NNT) to achieve an additional MCID response was calculated at Weeks 12/24 for UPA vs PBO and ADA vs PBO.
Results: Data from 1704 patients (UPA 15 mg: 429; UPA 30 mg: 423; PBO: 423; ADA: 429) were analyzed. At Week 12, both doses of UPA resulted in significant improvements from BL vs PBO across all PROs (Table 1). At Week 12, UPA 15 mg and 30 mg resulted in significant improvements from BL vs ADA in HAQ-DI, SAPS, and SF-36 physical component summary and UPA 30 mg vs ADA in 4 SF-36 domains (Figure 1). Compared with PBO, significantly more patients treated with UPA 15 mg and 30 mg reported improvements ≥ MCID in PtGA, pain, and HAQ-DI as early as Week 2 (first post-BL visit) that were maintained through Week 24. At Week 12, the proportions of patients reporting improvements ≥ MCID were significantly greater with both doses of UPA vs PBO across all PROs except SF-36 mental component summary (UPA 30 mg) with NNTs ranging from 3.0–11.4 for all PROs (Figure 2). The proportions of UPA-treated (both doses) patients reporting improvements ≥ MCID at Week 12 were similar to ADA-treated patients across most PROs and significantly higher than ADA-treated patients in HAQ-DI; improvements were maintained through Week 24.
Conclusion: Treatment with UPA 15 mg or UPA 30 mg resulted in clinically meaningful improvements in PROs vs PBO at 12 weeks in biologic DMARD-naïve patients with active PsA, which were maintained or further improved at Week 24. Overall, improvements were similar between UPA 15 mg and UPA 30 mg and improvements with both doses of UPA were similar or greater than those reported with ADA.
Medical writing services provided by Brandy Menges of JK Associates, Inc. (a member of Fishawack Group of Companies; Conshohocken, PA) and funded by AbbVie.
To cite this abstract in AMA style:
Strand V, Mease P, Soriano E, Kishimoto M, Salvarani C, Damjanov N, Anderson J, Blondell E, Zueger P, Saffore C, Gladman D. Improvement in Patient-Reported Outcomes in Patients with Psoriatic Arthritis with Inadequate Response to Non-Biologic DMARDs Treated with Upadacitinib versus Placebo or Adalimumab: Results from a Phase 3 Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/improvement-in-patient-reported-outcomes-in-patients-with-psoriatic-arthritis-with-inadequate-response-to-non-biologic-dmards-treated-with-upadacitinib-versus-placebo-or-adalimumab-results-from-a-pha/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvement-in-patient-reported-outcomes-in-patients-with-psoriatic-arthritis-with-inadequate-response-to-non-biologic-dmards-treated-with-upadacitinib-versus-placebo-or-adalimumab-results-from-a-pha/