Session Information
Date: Sunday, November 8, 2020
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Many patients with interstitial lung disease (ILD) have autoimmune features without a distinct rheumatic disease and are thus designated as having interstitial pneumonia with autoimmune features (IPAF). Rituximab (RTX) may be effective in IPAF, but relevant data are scarce. We describe 50 patients with IPAF treated with RTX at 2 large academic medical centers.
Methods: Patients age ≥18 years meeting the 2015 IPAF classification criteria and treated with rituximab were identified at two large academic medical centers, Mass General Brigham (MGB) and University of Chicago Medicine (UCM). At MGB, patients were identified using an institution-wide data repository of patients seen from 2000-2018. At UCM, patients were identified from an ILD registry between 2006-2019. Patients were excluded if they received RTX for a classifiable autoimmune disease or malignancy or if they had < 1 year of follow-up available. Clinical improvement was defined as meeting all 4 clinical domains at 1 year after RTX initiation: improvement in clinician assessment, decreased oxygen requirement, no hospitalization, and survival. Only those subjects with PFTs within 3 months prior to or 1 month after RTX initiation and 6-18 months after RTX initiation were included in the PFT analysis. PFT improvement was defined as ≥10% increase in forced vital capacity (FVC).
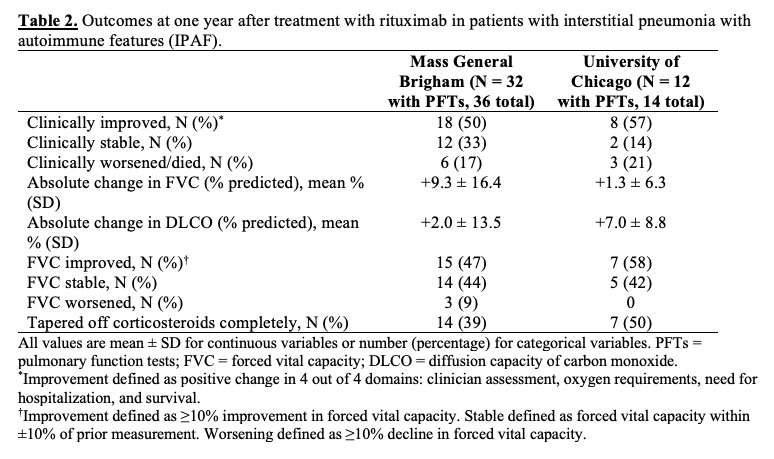
Results: At MGB, 36 IPAF patients (mean age 61 years, 44% female) were treated with RTX, with an average of 2.4 ± 1.3 cycles of RTX and 1.7 ± 0.6 doses per cycle (Table 1). At one year, 18 (50%) were clinically improved, 12 (33%) were stable, and 6 (17%) died from progressive respiratory failure (Table 2). Of 32 patients with PFTs available, the mean absolute change in FVC (% predicted) was +9.3% ± 16.4%. At UCM, 14 IPAF patients (mean age 53 years, 71% female) were treated with RTX with an average of 2.9 ± 2.1 cycles and 2.0 ± 0 doses per cycle. Compared to the MGB cohort, the UCM cohort was younger and had more females, Black/African American and Latinx patients, and baseline comorbidities. At the time of IPAF diagnosis, fewer patients at UCM required hospitalization for hypoxemia than at MGB (1 [7%] vs. 10 [28%]). In the UCM cohort at one year, 8 (57%) were improved, 2 (14%) were stable, and 3 (21%) died from progressive respiratory failure. Of 12 patients with PFTs available, the mean absolute change in FVC (% predicted) was +1.3% ± 6.3%. By one year, 14 (39%) patients in the MGB cohort and 7 (50%) in the UCM cohort had tapered off glucocorticoids completely. Overall at both sites, 9 patients had infections and 2 had minor infusion reactions; only 2 discontinued therapy due to adverse events (infections) during 1 year of therapy.
Conclusion: In 50 patients with IPAF treated with RTX at 2 large academic medical centers, most patients demonstrated improvement or stability at 1 year. These findings call for prospective studies, including randomized controlled trials, to further determine the risks, benefits, optimal timing, and cost effectiveness of RTX use in IPAF.
To cite this abstract in AMA style:
D'Silva K, Bauer Ventura I, Bolster M, Castelino F, Sharma A, Little B, Adegunsoye A, Strek M, Montesi S, Choi H. Rituximab for Interstitial Pneumonia with Autoimmune Features at Two Academic Medical Centers [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/rituximab-for-interstitial-pneumonia-with-autoimmune-features-at-two-academic-medical-centers/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/rituximab-for-interstitial-pneumonia-with-autoimmune-features-at-two-academic-medical-centers/