Session Information
Date: Sunday, November 8, 2020
Title: Epidemiology & Public Health Poster III: Inflammatory Rheumatic Disease
Session Type: Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose: Compared with the general population, patients with RA are at an increased risk of infection and certain malignancies, which may be increased further with the use of DMARDs.1,2 The abatacept global post-marketing epidemiology program included observational studies based on disease registries and healthcare claims databases. Reports from the core post-marketing epidemiology program on pre-specified outcomes associated with abatacept compared with other DMARD treatments in routine clinical practice showed no increase in the risk of infections requiring hospitalization or overall malignancies.3,4 The aim of this supplemental post-marketing abatacept only study was to evaluate the incidence of infections and pre-specified malignancies in patients from several countries with RA who were treated with abatacept.
Methods: Data were analyzed from seven RA registries: Anti-TNF Therapy in Rheumatoid Arthritis (ATTRA, Czech Republic), Danish Database for Biologic Therapies (DANBIO), National Registry of Biological Treatment in Finland (ROB-FIN), Swiss Clinical Quality Management (SCQM), Orencia and Rheumatoid Arthritis (ORA, France), The Italian Group for the Study of Early Arthritis (GISEA) and Spanish Registry of Adverse Events of Biological Therapies in Rheumatic Diseases (BIOBADASER). The first day of abatacept treatment was considered the index date and exposure was measured in patient-years (p-y). Incidence rates (IRs) with 95% CIs were calculated for each pre-specified outcome.
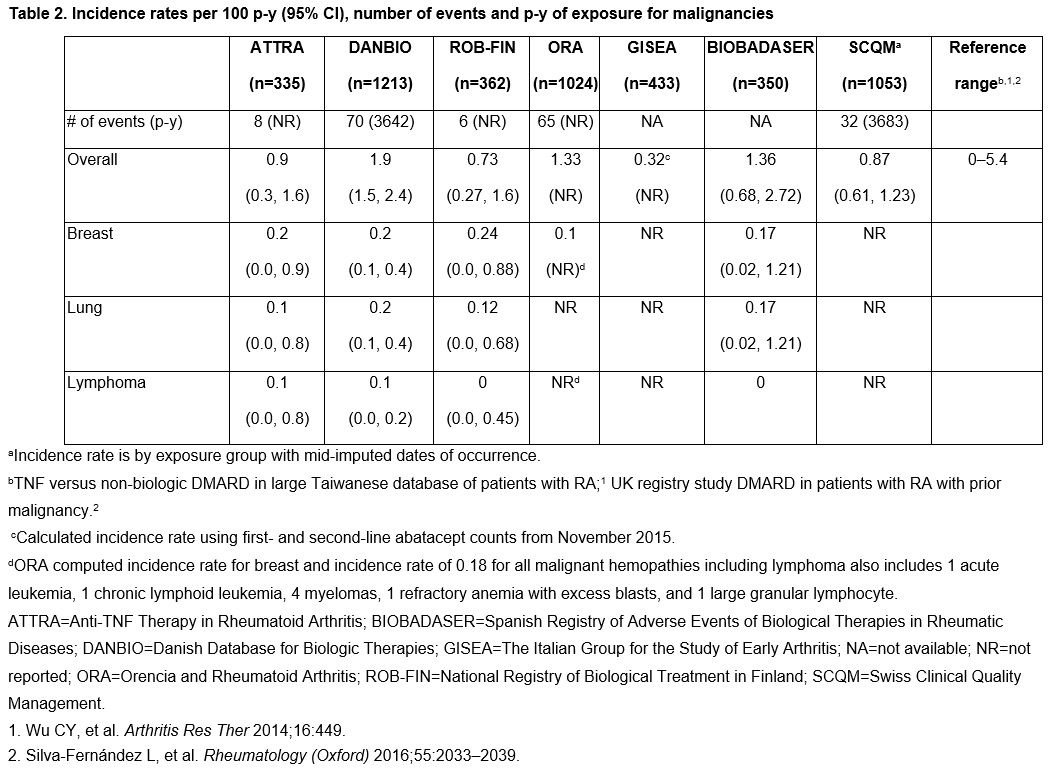
Results: Patients treated with abatacept ( >5000) were mostly female (78–85%), with a mean age ranging from 52–58 years. Baseline characteristics were largely consistent across the registries, except for longer disease duration in the ORA registry (mean [SD]: 20.4 [9.5] years; range across other registries: 8.1–13.3 years). In abatacept-treated patients, IRs for hospitalized infections across the registries varied, ranging from 0.4 to 10 per 100 p-y (Table 1). The IR for overall malignancies in abatacept-treated patients was low and ranged from 0.3 to 1.9 per 100 p-y (Table 2).
Conclusion: Despite the heterogeneity of the registries and possibility of under-reporting adverse events in observational studies, the safety profile of abatacept in this real-world observational study showed consistent findings in patients with RA treated with abatacept, with no new or increased risks of infection or malignancy identified.
References:
- Winthrop KL. Rheum Dis Clin North Am 2012;38:727–745.
- Simon TA, et al. Arthritis Res Ther 2015;17:212.
- Simon TA, et al. EULAR 2019. Poster FRI0118.
- Simon TA, at al. EULAR 2019. Oral OP0226.
Medical writing: Fiona Boswell (Caudex).
Contributor: Myriam Riek (SCQM Foundation, Zürich, Switzerland).
To cite this abstract in AMA style:
Dominique A, Hetland M, Finckh A, Gottenberg J, Iannone F, Caporali R, Nordstrom D, Hernandez M, Sanchez-Piedra C, Sanchez-Alonso F, Pavelka K, Křístková Z, Simon T. Infection and Malignancy Outcomes in Patients with RA Treated with Abatacept: Results from a Multinational Surveillance Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/infection-and-malignancy-outcomes-in-patients-with-ra-treated-with-abatacept-results-from-a-multinational-surveillance-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/infection-and-malignancy-outcomes-in-patients-with-ra-treated-with-abatacept-results-from-a-multinational-surveillance-study/