Session Information
Session Type: Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Gout’s high prevalence in kidney transplant (KT) recipients has been associated with heavy residual urate burden, decreased urate excretion related to reduced renal function, and high calcineurin inhibitor use in this population. The management of gout can be particularly challenging in KT patients due to decreased urate lowering therapy (ULT) renal clearance and drug-drug interactions. Pegloticase (pegylated recombinant uricase) is a treatment indicated for uncontrolled gout and tophi burden that rapidly metabolizes uric acid to allantoin which is readily cleared from the circulation. The use of pegloticase with immunomodulation therapy has been rising in order to reduce anti-drug antibody generation that decreases treatment efficacy and can increase risk of infusion reactions (IRs). Immunomodulator/pegloticase co-therapy markedly improves treatment responder rates1-3. However, only a few case reports4,5 of pegloticase use in solid organ transplant patients have been published and phase 3 pegloticase trials excluded organ transplant recipients. We conducted the PROTECT trial (NCT04087720) to examine pegloticase use in KT recipients with uncontrolled gout.
Methods: We are examining pegloticase treatment (12 biweekly 8 mg infusions over 24 weeks) in adult KT recipients (KT >1 year ago, eGFR ≥15 mL/min/1.73m2) with uncontrolled gout (serum uric acid [sUA] ≥7 mg/dL, ULT contraindication/inefficacy, and either tophi, chronic gouty arthritis, or ≥2 flares in past year). The primary endpoint is the proportion of pegloticase responders (sUA < 6 mg/dL for ≥80% of time during Month 6). We are also examining sUA levels, eGFR, urine albumin-to-creatinine ratio (UACR), and health assessment questionnaire (HAQ) pain scores.
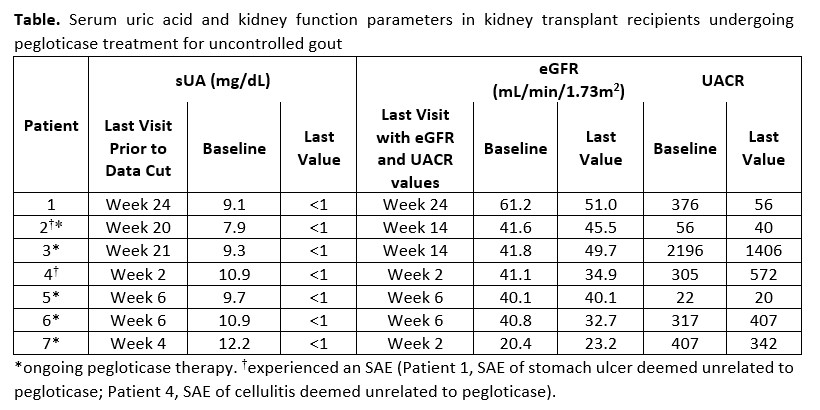
Results: At the time of this analysis (April 30, 2020), 7 patients who had undergone KT 15.3 ± 5.0 years earlier had enrolled (age: 52.0 ± 11.2 years, sUA: 10.0 ± 1.4 mg/dL, gout duration: 5.9 ± 4.3 years; COVID-19 reduced enrollment). All patients were on stable doses of ≥2 immunosuppressants. 1 patient had completed the 24-week treatment, 5 were in treatment, and 1 had discontinued early due to COVID-19 (non-infection) reasons (Table); the number of infusions received ranged from 2 to 12. In the 5 ongoing patients, all post Day 1 central lab sUA levels were < 1 mg/dL, indicative of treatment response. No notable changes in eGFR were observed; 2 patients with baseline albuminuria >300 mg/g showed >35% reduction in UACR by week 14. HAQ pain (0 to 100 VAS) decreased by 33.8 ± 2.7 (n=3) from baseline at week 20. 2 patients experienced flares; no IRs occurred. 2 SAEs (stomach ulcer, cellulitis), deemed unrelated to pegloticase, were reported.
Conclusion: Early findings of the ongoing PROTECT clinical trial suggest that pegloticase can safely and effectively be used in KT recipients to treat uncontrolled/refractory gout. Further efficacy and safety data in the KT population are pending.
References
- Albert J, et al. Arthritis Rheumatol 2019, 71(suppl 10).
- Masri K, et al. Ann Rheum Dis 2020, 79(suppl 1).
- Botson J, et al. Ann Rheum Dis 2020, 79(suppl 1).
- Freyne B. Transplant Proc 2018, 50:4099-101.
- Hershfield MS, et al. Arthritis Res Ther 2014, 16:R63.
To cite this abstract in AMA style:
Abdellatif A, Zhao L, Peloso P, Cherny K, Marder B, Scandling J, Saag K. A Multicenter, Open-Label, Efficacy and Safety Study of Pegloticase in Patients with Uncontrolled Gout Who Have Undergone Kidney Transplantation: Early Data Report [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/a-multicenter-open-label-efficacy-and-safety-study-of-pegloticase-in-patients-with-uncontrolled-gout-who-have-undergone-kidney-transplantation-early-data-report/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/a-multicenter-open-label-efficacy-and-safety-study-of-pegloticase-in-patients-with-uncontrolled-gout-who-have-undergone-kidney-transplantation-early-data-report/