Session Information
Date: Friday, November 6, 2020
Title: Spondyloarthritis Including Psoriatic Arthritis – Treatment I: Psoriatic Arthritis (0504–0508)
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: Lower efficacy to anti-tumor necrosis factor treatment has been reported in female patients with psoriatic arthritis (PsA) as compared to males in clinical registries.1 The EXCEED study (NCT02745080) evaluated efficacy and safety of secukinumab (SEC) versus adalimumab (ADA) as first-line monotherapy in biologic-naïve patients with PsA. Here, we report the impact of sex on efficacy outcomes with SEC versus ADA at Week 52 from the EXCEED study.
Methods: Eligible patients were randomized 1:1 to receive SEC 300 mg subcutaneous at baseline, Week 1-4, followed by dosing every 4 weeks until Week 48 or ADA 40 mg subcutaneous at baseline followed by same dosing every 2 weeks until Week 50. The detailed study design and objectives have been previously described.2 The primary analysis was to demonstrate superiority of SEC versus ADA on ACR20 response at Week 52 using logistic regression model with treatment as a factor and baseline weight as a covariate. A post-hoc analysis for ACR20 response was performed using the same model with treatment, sex and smoking status as factors to adjust for imbalances observed in the baseline characteristics. Comparative post–hoc analysis was performed with SEC versus ADA by sex for other efficacy outcomes through Week 52.
Results: A total of 853 patients were randomized to receive SEC (N=426) or ADA (N=427) with similar baseline demographics except for higher proportion of females (51% in SEC; 46% in ADA) and smokers (21·8% in SEC; 17·8% in ADA) in the SEC group.2 The primary endpoint for ACR20 response at Week 52 was not met. ACR20 response at Week 52 was, SEC (67·4%) versus ADA (61·4%); p=0·0227, after adjusting for sex and smoking status. The female patients had higher tender joint count, patient global assessment, HAQ-DI, PsA pain and enthesitis compared to males (Table 1). A greater proportion of patients with SEC (83·0% females; 91·3% males) completed Week 52 versus ADA (74·2% females; 83·4% males). SEC was associated with numerically higher efficacy on joints in females at Week 52 without any notable differences in the ACR core components versus ADA. Overall, higher efficacy was demonstrated in males versus females at Week 52 (Table 2).
Conclusion: Females had greater baseline disease severity than males. Secukinumab was associated with higher retention rate in both males and females at Week 52. Secukinumab provided numerically higher clinical responses in females versus adalimumab in musculoskeletal and skin endpoints and physical function at Week 52. In males, similar efficacy on musculoskeletal endpoints for both treatments and greater efficacy on skin with secukinumab were observed. These interesting findings warrant further investigation for reproducibility or uniqueness.
References
1. Højgaard P, et al. Rheumatology. 2018;57(9):1651-60.
2. McInnes IB, et al. Lancet. 2020;395:1496–505.
 Table 1: Baseline clinical characteristics
Table 1: Baseline clinical characteristics
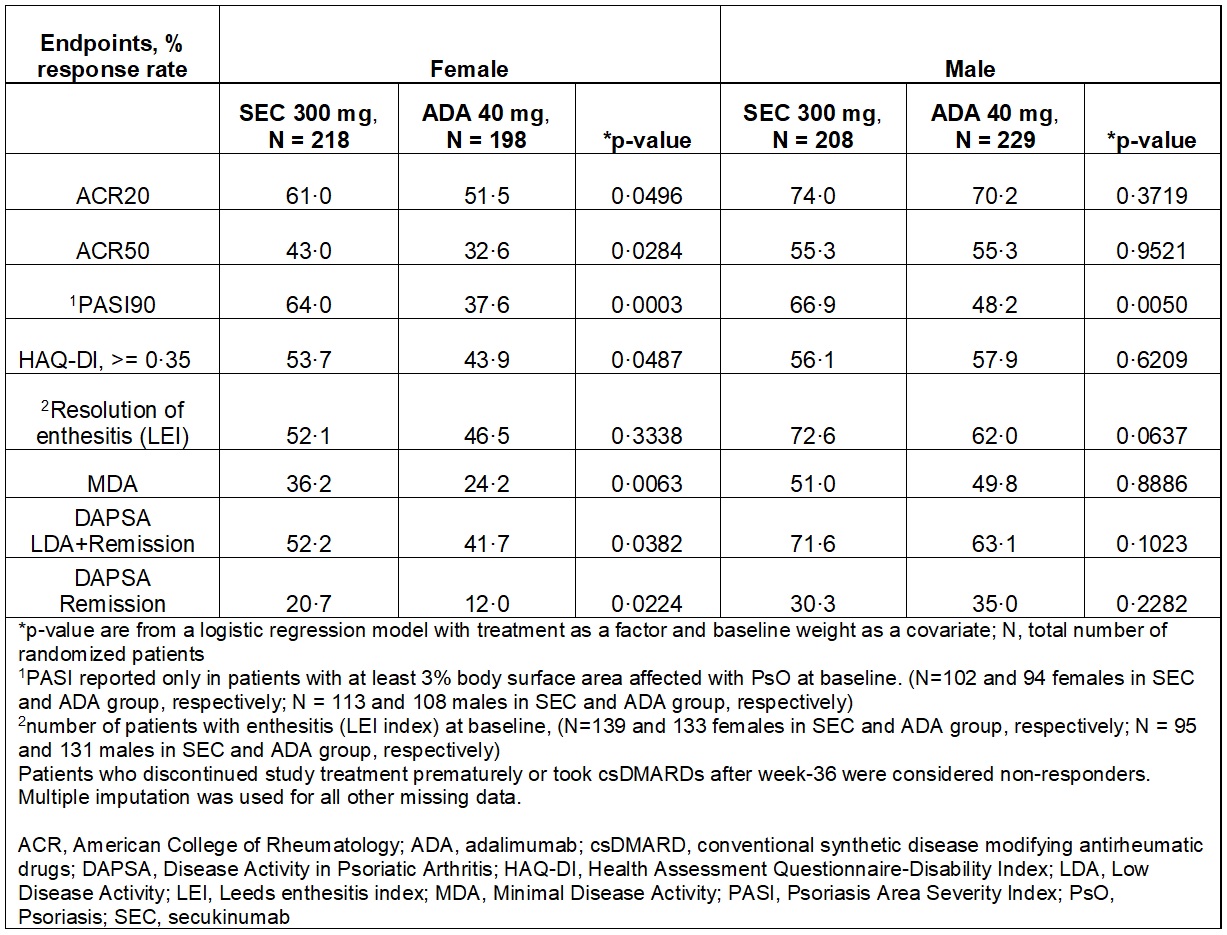 Table 2: Efficacy outcomes by sex at Week 52
Table 2: Efficacy outcomes by sex at Week 52
To cite this abstract in AMA style:
Wright G, Nash P, Coates L, Gratacós J, Behrens F, Ding K, Bao W, Pricop L, Gaillez C, McInnes I. Comparison of Secukinumab versus Adalimumab Efficacy by Sex in Psoriatic Arthritis from a Phase 3b, Double-blinded, Randomized, Active-controlled Study [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/comparison-of-secukinumab-versus-adalimumab-efficacy-by-sex-in-psoriatic-arthritis-from-a-phase-3b-double-blinded-randomized-active-controlled-study/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/comparison-of-secukinumab-versus-adalimumab-efficacy-by-sex-in-psoriatic-arthritis-from-a-phase-3b-double-blinded-randomized-active-controlled-study/
