Session Information
Session Type: Abstract Session
Session Time: 3:00PM-3:50PM
Background/Purpose: The recombinant herpes zoster vaccine (RZV) was FDA-approved in 2017 but patients with rheumatic diseases were excluded from initial pivotal trials because of theoretical risk of flares given the vaccine’s potent immunogenicity. Its use in patients with RA, SLE and vasculitis raises particular concern.
Methods: Patients followed in the Cleveland Clinic Rheumatology Department who received RZV between February 2018 and March 2020 were identified and retrospectively included in this study. Inclusion criteria were: age > 18 years, at least one RZV dose during the study period, available data on the clinical status before and after the vaccine. Data were collected from the electronic medical record. An immune mediated inflammatory disease (IMID) flare was defined as a) a documentation of flare in the rheumatology office notes, telephone encounter or patient portal communication or b) new prednisone prescription, occurring in the 12 week period following each dose. Adverse events (AE) attributed to RZV and occurrence of herpes zoster (HZ) were also assessed. The statistical analysis was performed with R studio v.1.2.5042. Categorical variables were compared using chi-square tests and multivariable analysis were performed using logistic regression and Cox model (tests were two-sided, significance level was 0.05).
Results: In the study period, 622 patients met the inclusion criteria; the majority were female (67%), and median age was 67 years. Of the group, 15% reported a history of HZ and 43% had previously received live attenuated zoster vaccine. 147 patients (24%) received 1 dose of RZV, while 475 (76%) received both dsoses with a median interval of 2.9 months. The AE rate was 8.5% with mostly mild events (71%). After a median 35.8 weeks of follow-up, the HZ incidence was 0.6%.
Of 358 IMID patients the most common were RA (n=88, 25%), vasculitis (n=50, 14%), and PMR (n=29, 8%). Baseline treatment in this group included glucocorticoids (GC) (35%, median dose 5 mg/day), conventional and biologic DMARDs (Fig 1). Fifty-seven (16%) experienced a flare: 34 (60%) after the first dose, 16 (28%) after the second, and 7 (12%) flared after both. RA patients had the highest flare rate (n=21, 24%). Flares occurred in temporal relation to a treatment change in 18 cases (31%). Flares were most often treated with GC (44%, median dose 20 mg/day) and 14 (25%) required a change in immunosuppressive therapy. Among IMID patients, univariate analysis revealed more flares in patients on GC and Jak-inhibitors (p=0.002 and 0.025 respectively), and in RA patients (p=0.019) (Table 1). A multivariate analysis showed that only GC use at time of vaccine remained significantly associated with flares (OR = 2.31 [1.29-4.15], p=0.005). A time-to-flare survival analysis was conducted with a multivariate Cox-model: glucocorticoids remained the only significant predictor of the first IMID flare (HR = 2.34 [1.3-4.3], p=0.006) (Fig 2).
Conclusion: In this retrospective cohort of rheumatic disease patients, we found the incidence of AEs and HZ after RZV to be consistent with the existing literature. GC at time of RZV was associated with a significantly higher rate of flares, suggesting that delaying RZV administration in patients with active disease may be prudent
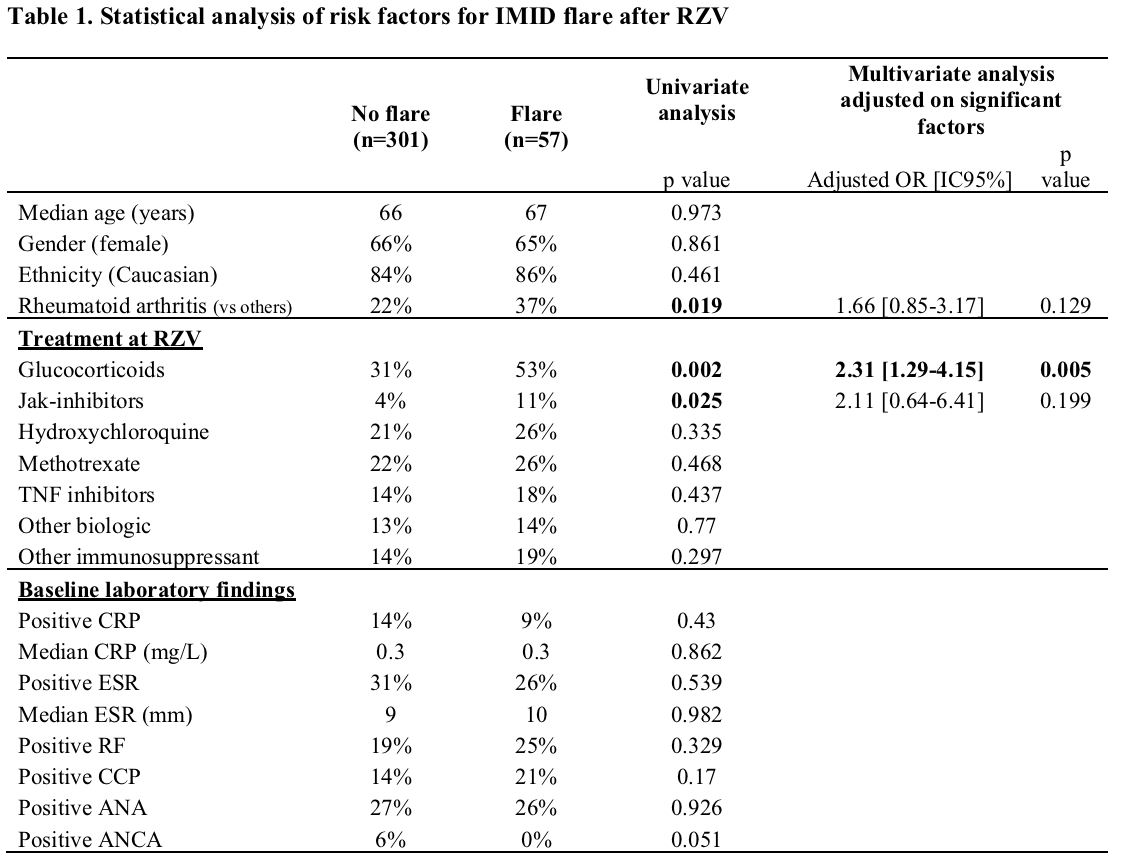 Legend: IMID: Immune Mediated Inflammatory Disease; RZV: Recombinant Zoster Vaccine; OR: Odd-ratio; IC: Confident Interval
Legend: IMID: Immune Mediated Inflammatory Disease; RZV: Recombinant Zoster Vaccine; OR: Odd-ratio; IC: Confident Interval
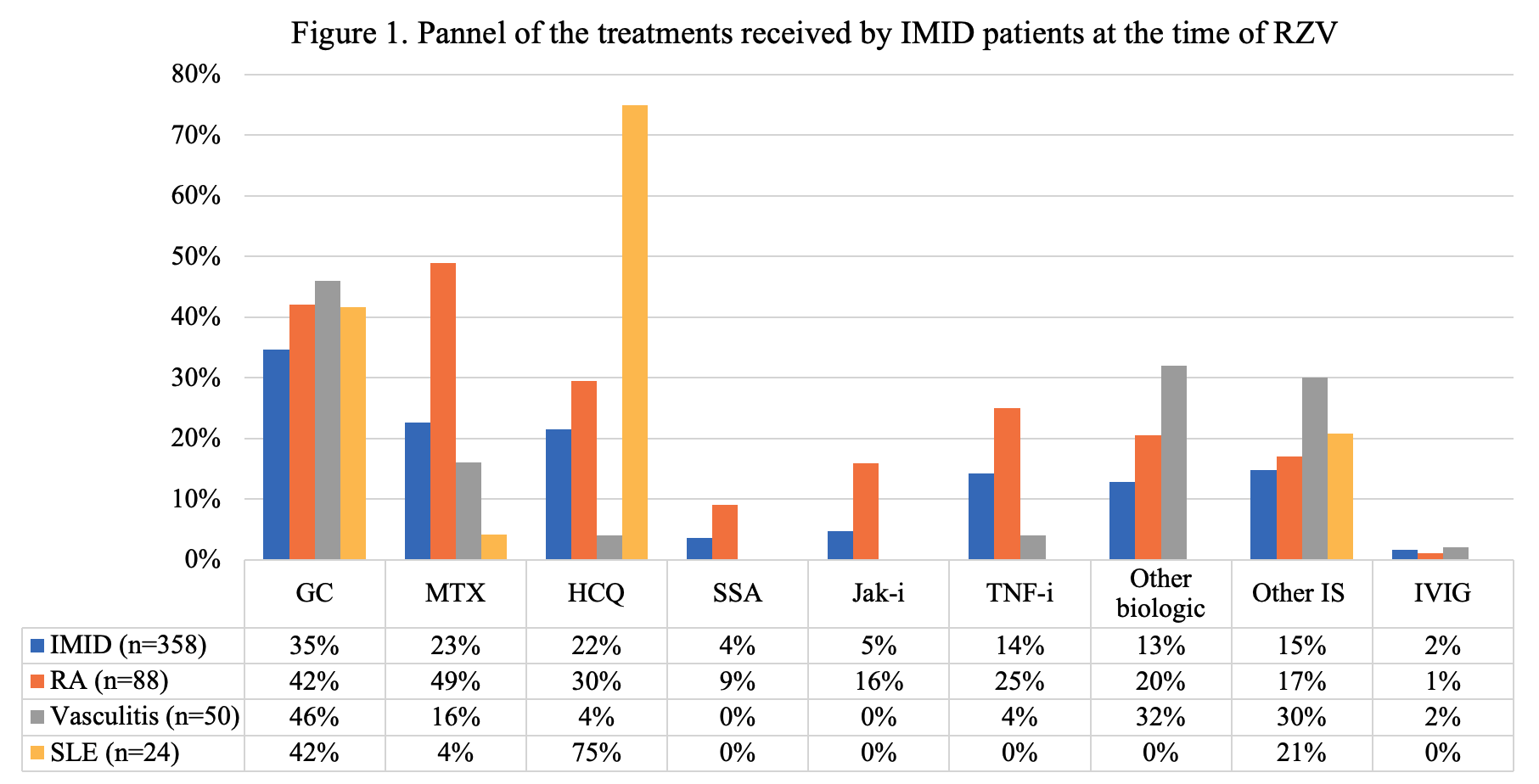 Legend. IMID: Immune Mediated Inflammatory Disease; RZV: Recombinant Zoster Vaccine; RA: Rheumatoid Arthritis; SLE: Systemic Lupus Erythematosus; GC: Glucocorticoids; MTX: Methotrexate; HCQ: Hydroxychloroquine; SSA: Sulfasalazine; Jak-i: Jak-inhibitors; TNF-i: TNF inhibitors; Other biologic: abatacept, apremilast, belimumab, mepolizumab, rituximab, secukinumab, tocilizumab, ustekinumab; Other IS: Other Immunosuppressant, azathioprine, leflunomide, ciclosporine, mycophenolate mofetil, tacrolimus; IVIG: Intravenous Immunoglobulin.
Legend. IMID: Immune Mediated Inflammatory Disease; RZV: Recombinant Zoster Vaccine; RA: Rheumatoid Arthritis; SLE: Systemic Lupus Erythematosus; GC: Glucocorticoids; MTX: Methotrexate; HCQ: Hydroxychloroquine; SSA: Sulfasalazine; Jak-i: Jak-inhibitors; TNF-i: TNF inhibitors; Other biologic: abatacept, apremilast, belimumab, mepolizumab, rituximab, secukinumab, tocilizumab, ustekinumab; Other IS: Other Immunosuppressant, azathioprine, leflunomide, ciclosporine, mycophenolate mofetil, tacrolimus; IVIG: Intravenous Immunoglobulin.
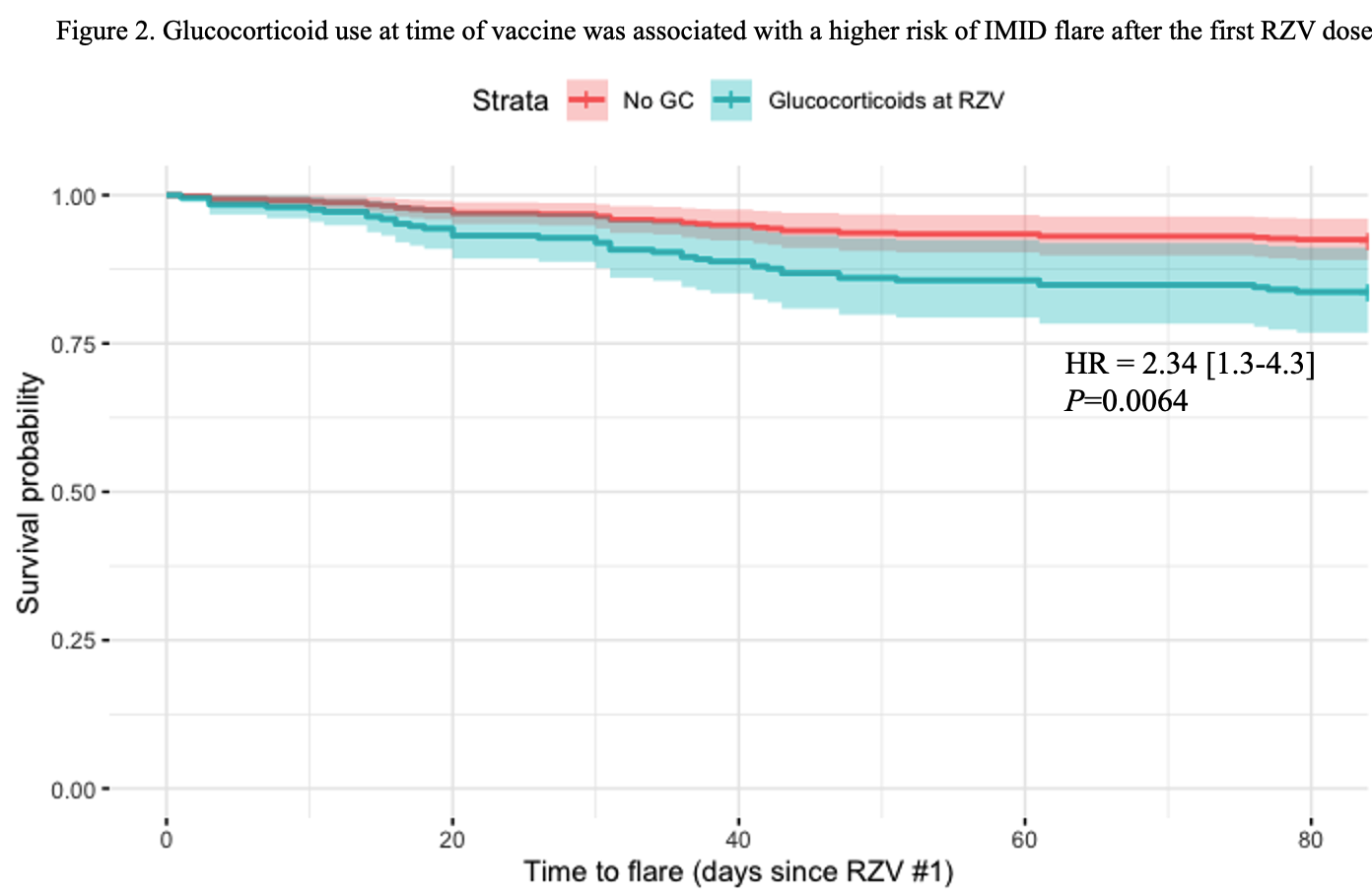 Legend: This figure shows a survival analysis of the time-to-flare with estimated risk of being on glucocorticoids at the time of first RZV dose (multivariate Cox-model). IMID: Immune Mediated Inflammatory Disease; RZV: Recombinant Zoster Vaccine; GC: Glucocorticoids; HR: Hazard Ratio
Legend: This figure shows a survival analysis of the time-to-flare with estimated risk of being on glucocorticoids at the time of first RZV dose (multivariate Cox-model). IMID: Immune Mediated Inflammatory Disease; RZV: Recombinant Zoster Vaccine; GC: Glucocorticoids; HR: Hazard Ratio
To cite this abstract in AMA style:
Lenfant T, Kirchner E, Hajj-ali R, Calabrese L, Calabrese C. Recombinant Zoster Vaccine in Patients with Rheumatic Diseases: A Retrospective Study of 622 Patients [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/recombinant-zoster-vaccine-in-patients-with-rheumatic-diseases-a-retrospective-study-of-622-patients/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/recombinant-zoster-vaccine-in-patients-with-rheumatic-diseases-a-retrospective-study-of-622-patients/
