Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Within the Anti-Nuclear Antibody (ANA) associated Systemic Autoimmune Rheumatic Diseases (SARD), such as Systemic Lupus Erythematous (SLE), Sjögren’s Syndrome (SS), and Systemic Sclerosis (SSc), changes in the quantity and/or abundance of specific auto-antibodies (auto-Ab) occur as individuals progress towards SARD. However, previous studies have examined only a small number of ANAs. Thus, additional changes in the auto-Ab profile may accompany and predict disease progression that have not yet been appreciated. To address this, we examined the auto-Ab profile for a broad range of auto-antigens (auto-Ag) across 245 ANA+ asymptomatic and symptomatic individuals.
Methods: A microarray was used to simultaneously measure 144 auto-Ab specificities across six patient groups: ANA– healthy controls (HC, n=38); ANA+ (≥1:160 by IF) individuals lacking SARD criteria (ANA+ NS, n=83); individuals with ≥ 1 SARD criteria but with insufficient evidence for a diagnosis (undifferentiated connective tissue disease; UCTD, n=52); early untreated (except anti-malarial) SARD patients (n=26 SLE, n=30 SS, and n=16 SSc, classified according to the 1997 ACR criteria, 2013 ACR-EULAR criteria, and the 2016 ACR-EULAR criteria, respectively). ANA+ NS and UCTD patients were followed yearly for up to 4 years, 12 of which demonstrated symptom progression. Eleven specific ANAs were detected using the Bioplex 2200 ANA-Screen and the expression levels of five IFN-α induced genes were measured and summed to generate an IFN5 score.
Results: Following unsupervised hierarchical clustering, IgG but not IgM auto-Ab showed clear patterns by diagnosis (heat map for IgG shown in Figure 1). A subset of ANA+ NS and UCTD individuals were admixed with SLE and SS patients, which included the majority of progressors. In general, there was a moderate positive correlation between the levels of the auto-Ab detected by Bioplex and the corresponding auto-Ab in the microarray, however, using 2 standard deviations above the mean for ANA– HC as a cut-off, elevated levels of auto-Ab were seen more frequently in ANA+ individuals by microarray than by Bioplex. Furthermore, the microarray was able to detect differences in auto-Ab levels above the upper limit of detection on Bioplex, with very high levels restricted to SARD patients (Figure 2). Many more auto-Ag were recognized by IgG auto-Ab in SLE than in the other ANA+ groups indicating that self-tolerance is significantly more disrupted in SLE (Figure 3). Interestingly, although anti-Ro60 had the strongest correlation with IFN-induced gene expression, only the presence of IgG anti-Ro52 at baseline was associated with progression in ANA+ individuals lacking a SARD diagnosis. Strikingly, the types and levels of auto-Abs remained remarkably stable over time in these individuals regardless of whether they progressed or not.
Conclusion: ANA+ individuals have auto-Ab to many self-antigens that are not being captured by current screening techniques. Measurement of these additional auto-Ab specificities, together with the larger dynamic range of the microarray, may help to identify individuals at high risk of progression.
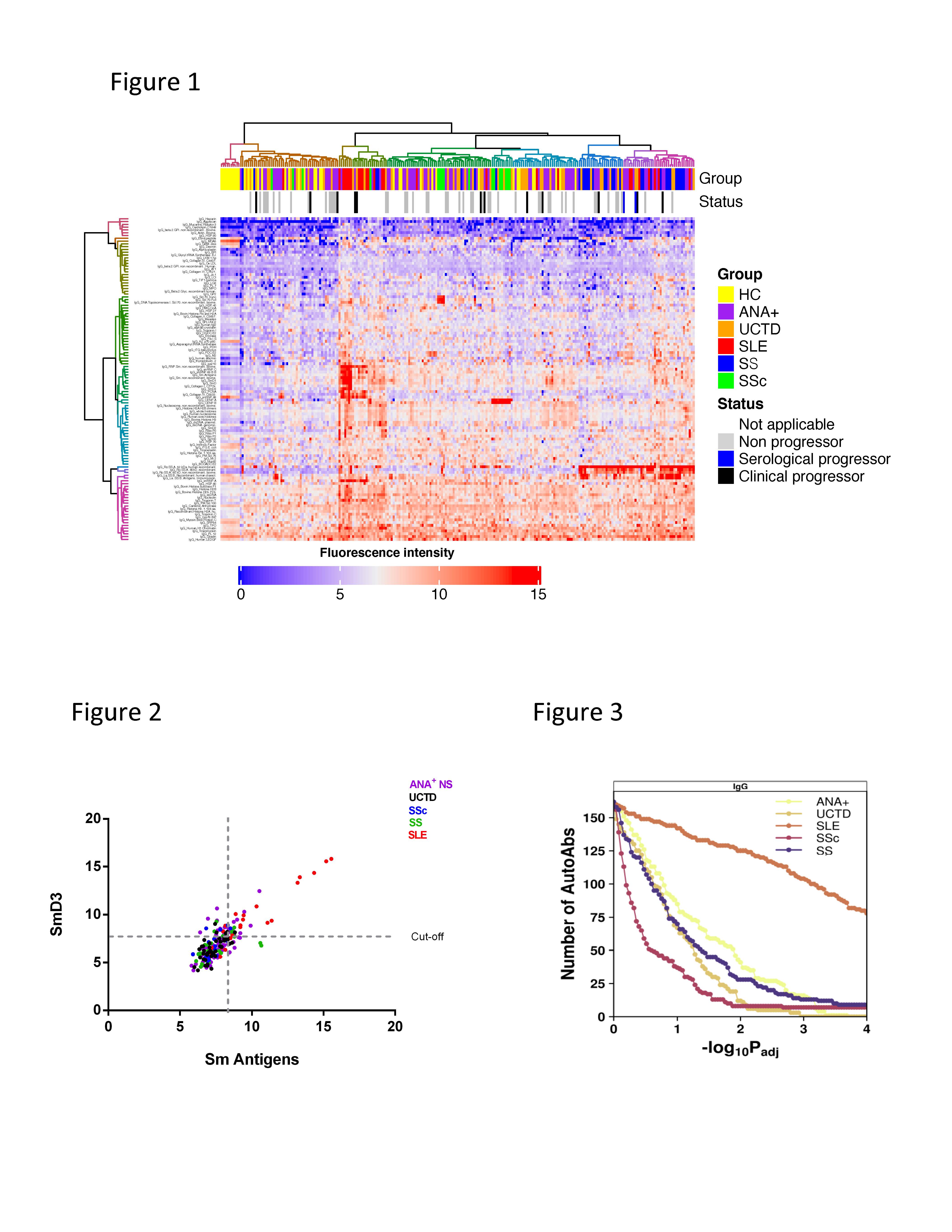 Figure 1. Heat Map of selected antigens following unsupervised clustering. IgG auto-Ab levels in red are high and those in blue are low. Controls are clustered on the left. Note the admixture of asymptomatic ANA+ and UCTD individuals with the early SARD patients, indicating similar auto-Ab profiles. Figure 2. Representative plot showing the levels of auto-Ab (Sm antigen and SmD3 subcomponent in this example) determined by microarray. Note that very high levels are restricted to SLE patients. Figure 3. P-value sensitivity plot examining the number of significantly differentially abundant probes relative to healthy controls, where it can be appreciated that many more IgG auto-Abs are elevated in SLE than in the other ANA+ groups.
Figure 1. Heat Map of selected antigens following unsupervised clustering. IgG auto-Ab levels in red are high and those in blue are low. Controls are clustered on the left. Note the admixture of asymptomatic ANA+ and UCTD individuals with the early SARD patients, indicating similar auto-Ab profiles. Figure 2. Representative plot showing the levels of auto-Ab (Sm antigen and SmD3 subcomponent in this example) determined by microarray. Note that very high levels are restricted to SLE patients. Figure 3. P-value sensitivity plot examining the number of significantly differentially abundant probes relative to healthy controls, where it can be appreciated that many more IgG auto-Abs are elevated in SLE than in the other ANA+ groups.
To cite this abstract in AMA style:
Munoz-grajales C, Prokopec S, Bonilla D, Silverman E, Johnson S, Bookman A, Touma Z, Ahmad Z, Hiraki L, Boutros P, Chruscinski A, Wither J. Investigating the Differences in ANA Specificities Between Asymptomatic and Symptomatic ANA+ Individuals [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/investigating-the-differences-in-ana-specificities-between-asymptomatic-and-symptomatic-ana-individuals/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/investigating-the-differences-in-ana-specificities-between-asymptomatic-and-symptomatic-ana-individuals/
