Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Iron is vital for many physiological processes and is found within respiratory complexes of the mitochondrial electron transport chain, the key site of oxidative phosphorylation ATP energy production.
Previously we demonstrated that patients with SLE are at risk of absolute Iron deficiency, which occurs when stores of iron within the body become deplete. Patients are also at risk of a state of functional iron deficiency, in which there is adequate supplies of iron within the body but there is an inability to mobilise or release iron from stores. At a cellular level, upregulation of the transferrin receptor is consistent with cellular iron deficiency and this has not previously been studied in SLE.
Given that preliminary findings suggest patients are at risk of iron deficiency, this study aimed to identify evidence of cellular iron deficiency and study the impacts on mitochondrial function.
Methods:
- Investigating for evidence of cellular iron deficiency and changes in mitochondrial mass in SLE. Flow cytometry was used to identify cell surface lineage markers of B-cells, T-cells and Monocytes. Cellular transferrin receptor was measured using median fluorescence intensity to quantify cell surface expression of the key iron receptor. Mitochondrial mass was measured using the dye MitoTracker Green.
- Studying mitochondrial function in iron deficiency and SLE. Peripheral blood mononuclear cells (PBMCs) from healthy controls (HCs) and patients with SLE were analysed using Seahorse Respirometry, which measures mitochondrial oxygen consumption rate (a measure of energy metabolism dependent upon oxidative phosphorylation). To assess differences between health, iron deficiency and SLE, 3 groups were assessed; 1. PBMCs derived from HCs; 2. PBMCs from patients with SLE; 3. Healthy PBMCs cultured in iron deficient condition, in which cells were treated with the potent iron chelator, Deferiprone. Following analysis of PBMCs, magnetic bead isolation was used to specifically study monocytes (CD14+) and T-helper cells (CD4+).
Results: Figure 1 highlights that SLE CD4+ T-cells, monocytes and B-cells show increased cell surface transferrin receptor expression compared with HCs thus suggesting these cells are iron deficient.
Figure 2a demonstrates that PBMCs derived from healthy controls grown in iron deficient conditions (with Deferiprone) show both basal and maximal mitochondrial respiration is reduced. PBMCs derived from patients with SLE show similarly reduced respiration at a level comparable to iron deficient healthy cells. Figure 2b shows that monocytes from patients with SLE have reduced basal and maximal respiration when compared with those from HCs whilst Figure 2c shows that T-cells show reduced basal but not maximal respiration.
Figure 3 reveals that both CD4+ and CD8+ T-cells have significantly lower mitochondrial mass when compared with HCs.
Conclusion: These findings suggest that patients with SLE display evidence of cellular iron deficiency in a number of immune cells. In addition, CD4+ T-cells and monocytes derived from SLE patients show reduced mitochondrial respiration and T-cells from patients with SLE have lower mitochondrial mass, which may be a result of mitochondrial damage due to iron deficiency.
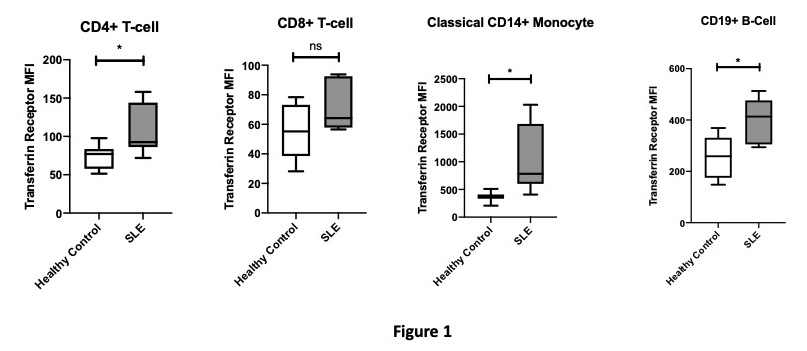 Figure 1: Transferrin Receptor Expression
Figure 1: Transferrin Receptor Expression
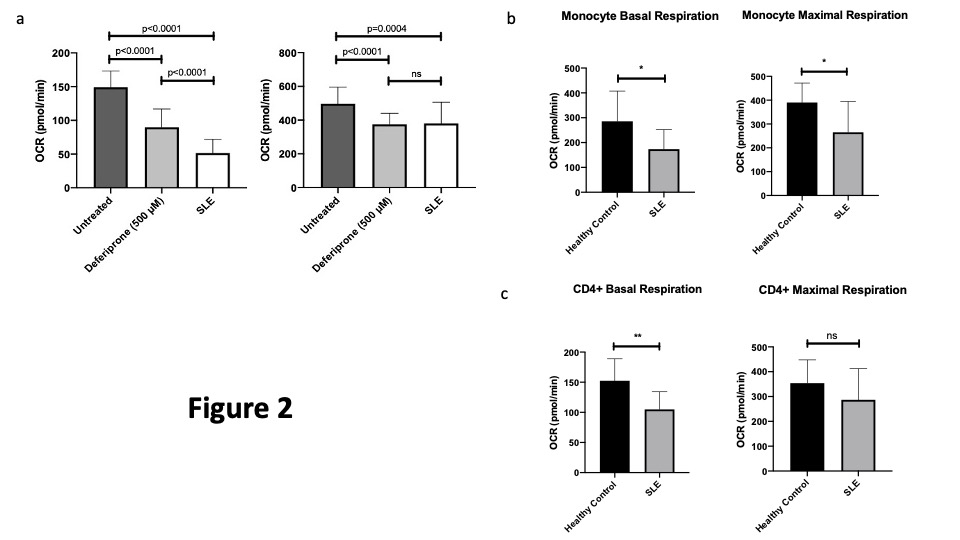 Figure 2: Basal and Maximal Mitochondrial Respiration
Figure 2: Basal and Maximal Mitochondrial Respiration
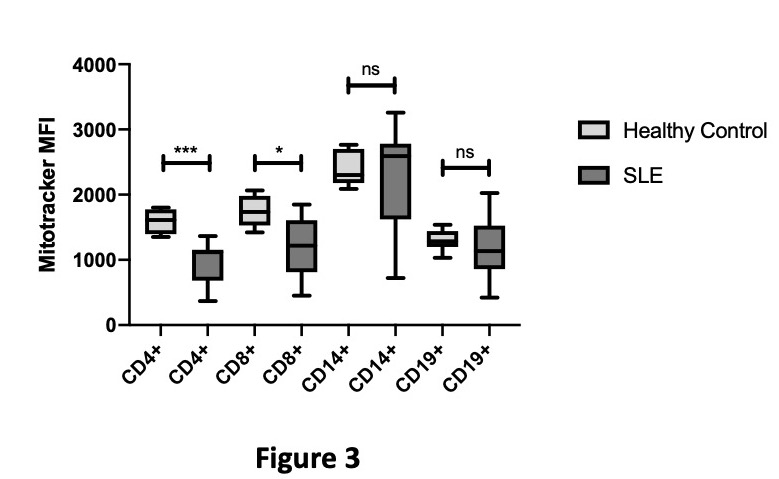 Figure 3: Differences in Mitochondrial Mass between Healthy Controls and SLE
Figure 3: Differences in Mitochondrial Mass between Healthy Controls and SLE
To cite this abstract in AMA style:
Wincup C, McDonnell T, Robinson G, Farinha F, Radziszewska A, Rahman A. Tired T-Cells and Monocytes with Malaise: Investigating the Links Between Cellular Iron Deficiency and Mitochondrial Dysfunction in Systemic Lupus Erythematosus [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/tired-t-cells-and-monocytes-with-malaise-investigating-the-links-between-cellular-iron-deficiency-and-mitochondrial-dysfunction-in-systemic-lupus-erythematosus/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/tired-t-cells-and-monocytes-with-malaise-investigating-the-links-between-cellular-iron-deficiency-and-mitochondrial-dysfunction-in-systemic-lupus-erythematosus/
