Session Information
Session Type: Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: The safety and efficacy of upadacitinib (UPA), an oral JAK inhibitor, was evaluated in the phase 3 SELECT clinical program, which included 5 randomized, double-blind, controlled trials across a spectrum of rheumatoid arthritis (RA) patients (pts)1-5. We describe the long-term integrated safety profile of UPA relative to active comparators in pts with RA treated in the SELECT program up to a cut-off date of 30 June 2019.
Methods: Treatment-emergent adverse events (TEAEs: AE onset ≥first dose and ≤30 days after last dose) were summarized for the following: methotrexate (MTX, 1 trial, mean exposure 76 wks); adalimumab (ADA, 1 trial, mean exposure 69 wks); pooled UPA 15 mg (5 trials, mean exposure 90 wks); pooled UPA 30 mg (4 trials, mean exposure 100 wks). TEAEs are reported as exposure-adjusted event rates (EAERs; events/100 patient years [E/100PYs]).
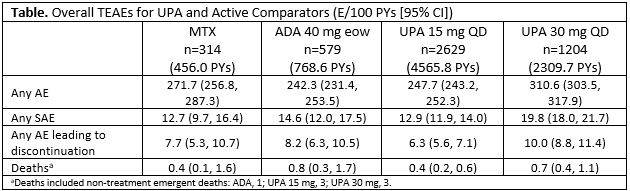
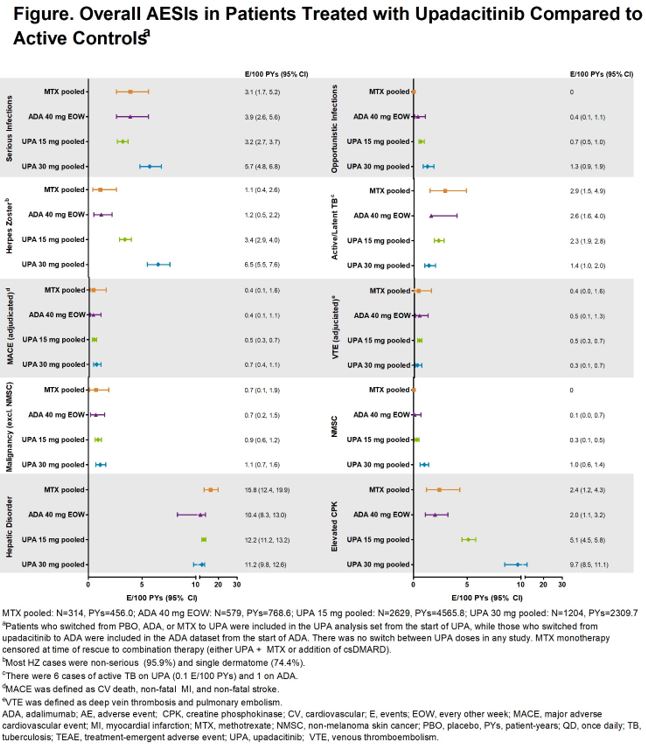
Results: 3833 pts received ≥1 dose of UPA 15 mg [n=2629, 4565.8 PYs] or 30 mg [n=1204, 2309.7 PYs] QD, with no option to switch doses. More than half of pts received UPA for ≥96 wks (median: UPA 15, 101.9 wks; UPA 30: 111.7 wks). The EAERs of overall SAEs and AEs leading to discontinuation on UPA 15 mg were comparable to MTX and ADA; rates on UPA 30 mg were numerically higher than UPA 15 mg (Table). The most common AEs (≥5 E/100 PYs) reported with UPA 15 mg were upper respiratory tract infection (URTI), nasopharyngitis, urinary tract infection (UTI), bronchitis, increased CPK, and increased ALT. For UPA 30 mg, the most common AEs reported were URTI, UTI, increased CPK, nasopharyngitis, bacterial bronchitis, and herpes zoster (HZ). Overall rates of serious infections and opportunistic infections were comparable between UPA 15 mg, MTX, and ADA groups but were higher on UPA 30 mg (Figure). Rates of HZ were higher in both UPA groups (30 mg higher than 15 mg) vs MTX and ADA. The majority of HZ cases were non-serious (96%) and involved a single dermatome (74%). Rates of VTE were comparable across treatment groups (0.3-0.5/100 PYs), as were rates of adjudicated MACE and malignancies (excluding NMSC). Rates of NMSC in UPA 15 mg and ADA were similar, with numerically higher rates on UPA 30 mg. SMR analysis demonstrated that the number of deaths in pts with RA exposed to UPA was not higher than what would be expected for the general population.
Conclusion: Through long-term follow-up, the integrated safety profile of UPA remained consistent with previous analyses, with no new signals identified.
REFERENCES:
1. Burmester, et al. Lancet 2018;391:2503-12.
2. Genovese, et al. Lancet 2018;391:2513-24.
3. Smolen, et al. Lancet 2019;393:2303-11.
4. Fleischmann, et al. Arthritis Rheumatol 2019;71:1788-1800.
5. van Vollenhoven, et al. Arthritis Rheumatol 2018;70(Suppl 10).
Original abs: Ann Rheum Dis. 2020; 79(S1):315.
To cite this abstract in AMA style:
Cohen S, Van Vollenhoven R, Curtis J, Calabrese L, Zerbini C, Tanaka Y, Bessette L, Schlacher C, Shaw T, Liu J, Enejosa J, Song Y, Burmester G. Safety Profile of Upadacitinib up to 3 Years of Exposure in Patients with Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2020; 72 (suppl 10). https://acrabstracts.org/abstract/safety-profile-of-upadacitinib-up-to-3-years-of-exposure-in-patients-with-rheumatoid-arthritis/. Accessed .« Back to ACR Convergence 2020
ACR Meeting Abstracts - https://acrabstracts.org/abstract/safety-profile-of-upadacitinib-up-to-3-years-of-exposure-in-patients-with-rheumatoid-arthritis/