Session Information
Date: Tuesday, November 12, 2019
Title: 5T093: SLE – Clinical V: Emerging Knowledge of Current Treatments (2780–2785)
Session Type: ACR Abstract Session
Session Time: 2:30PM-4:00PM
Background/Purpose: SLE is typified by a wide spectrum of clinical manifestations and immune dysregulation. Corticosteroids are almost universally effective, but marked by unacceptable side effects. An understanding of steroid efficacy signals may provide clues for safer, more targeted treatments.
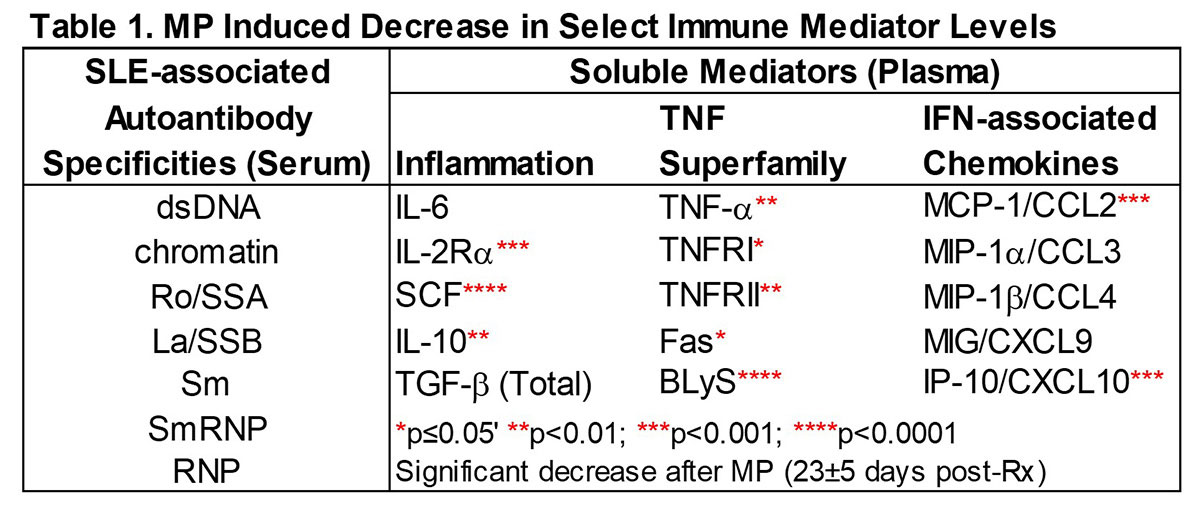
Methods: This study capitalized on entry samples from the Phase 2 XmAb®5871 study to define which immune effectors are sensitive to methylprednisolone (MP). Plasma immune mediators and serum autoantibodies (AutoAbs) were evaluated by xMAP assay in 103 active SLE pts (≥ 4 ACR criteria) assessed pre- and post-MP Rx vs. race/sex/age-matched controls (n=78). Fifty-two patients were randomized to continue the study on placebo, and were serially followed for 191 ± 75 days for changes in clinical disease activity (by hybrid SLEDAI [hSLEDAI]). Biomarkers (Table 1) were measured in an average of 5 serially collected plasma samples (range 3-6, average of 45 days between sample visits). The advantage of this BOLD trial design was the control of MP Rx by intramuscular injection and required withdrawal of background immunosuppressants other than HCQ and ≤ 10 mg prednisone/equivalent/day.
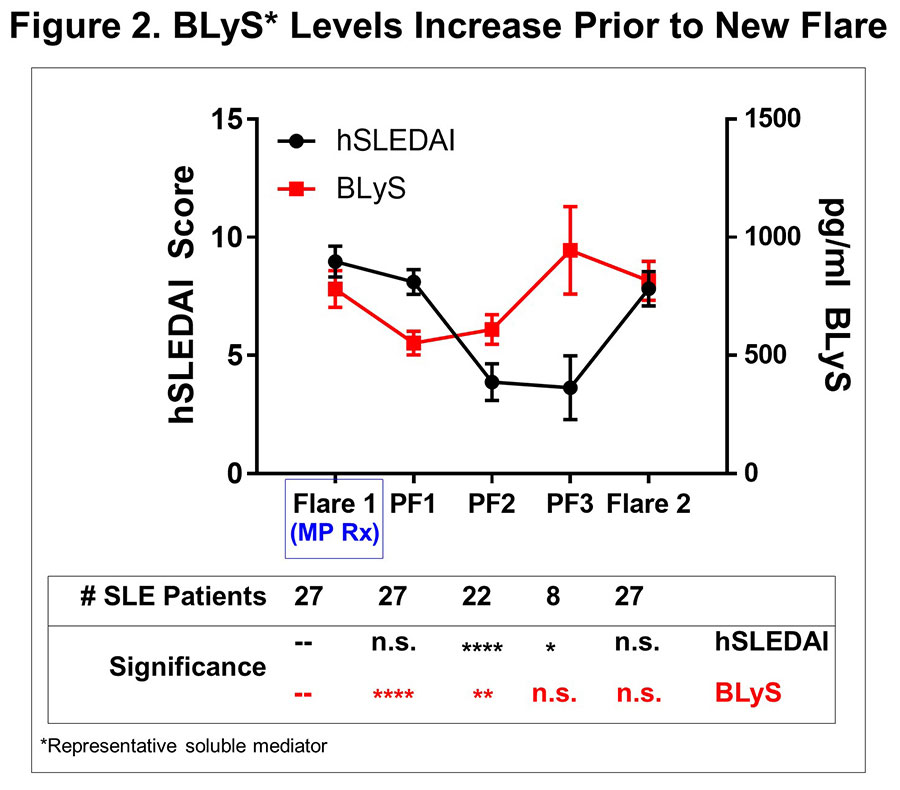
Results: MP resulted in decreased levels of a number of immune mediators (all p≤0.02, Table 1) that were readily detected in SLE plasma and higher in cases vs. ctls (p< 0.0001); BLyS and Stem Cell Factor (SCF) levels were most affected (p< 0.0001). This occurred prior to or in conjunction with decreased hSLEDAI scores (p=0.0001), decreased arthritis (p=0.0471), and decreased serologic (p=0.0003) features (Figure 1). Of 52 placebo pts with serial samples, 49 exhibited clinical disease flare at one or more visits, primarily due to increased arthritis and mucocutaneous features. When we evaluated samples at time of clinical disease flare and up to three post-flare samples thereafter in aggregate (Figure 1), we noted that specific immune mediators, including TNF-α, TNFRII, SCF, IL-2Rα, MCP-1, IP-10, and BLyS transiently decreased (all p≤0.03) in the first post-flare sample (PF1).These same mediators then increased again to clinical flare levels in PF2 and PF3 samples, preceding clinical evidence of new disease flares (Figure 2). Within the 145 ± 75 days between flares, there was little to no effect on number or type of SLE-associated AutoAbs (Figure 1).
Conclusion: Changes in a distinct subset of immune mediators are sensitive to MP Rx and were observed before or with hSLEDAI improvement. Subsequent resurgence of BLyS, TNF-α, TNFRII, SCF, IL-2Rα, MCP-1, and IP-10 preceded new clinical disease flares. This study underscores the probable utility of these markers for early intervention before clinical disease flares.
To cite this abstract in AMA style:
Munroe M, Guthridge C, Kleckner S, Tran L, Guthridge J, Zack D, James J, Merrill J. Alterations in Inflammatory, TNF-Superfamily, and IFN-Associated Chemokines Precede Clinical Changes in SLEDAI After Methylprednisolone Treatment of SLE Patients [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/alterations-in-inflammatory-tnf-superfamily-and-ifn-associated-chemokines-precede-clinical-changes-in-sledai-after-methylprednisolone-treatment-of-sle-patients/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/alterations-in-inflammatory-tnf-superfamily-and-ifn-associated-chemokines-precede-clinical-changes-in-sledai-after-methylprednisolone-treatment-of-sle-patients/