Session Information
Date: Tuesday, November 12, 2019
Title: Pediatric Rheumatology – ePoster III: Systemic JIA, Fever, & Vasculitis
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Down syndrome arthropathy (DA) is under-recognized with a 19-month average delay in diagnosis (1). The majority present with polyarticular, rheumatoid factor (RF) and anti-nuclear antibody (ANA) negative disease. The current therapies for juvenile idiopathic arthritis (JIA) appear to be poorly tolerated, more toxic and less effective in patients with DA (1). The objective of this study was to characterize clinical manifestations and therapeutic preferences in DA, using the new Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry.
Methods: The new CARRA Registry began prospectively collecting data on children with JIA in the United States and Canada in July 2015. Down syndrome (DS) is documented in the CARRA Registry as a coexisting condition. Within the new CARRA Registry, between the dates of July 2015 and March 2019, patients with JIA and DS were identified and an observational cohort analysis was performed. Collected data included demographics, disease characteristics, laboratory results, and treatment exposure.
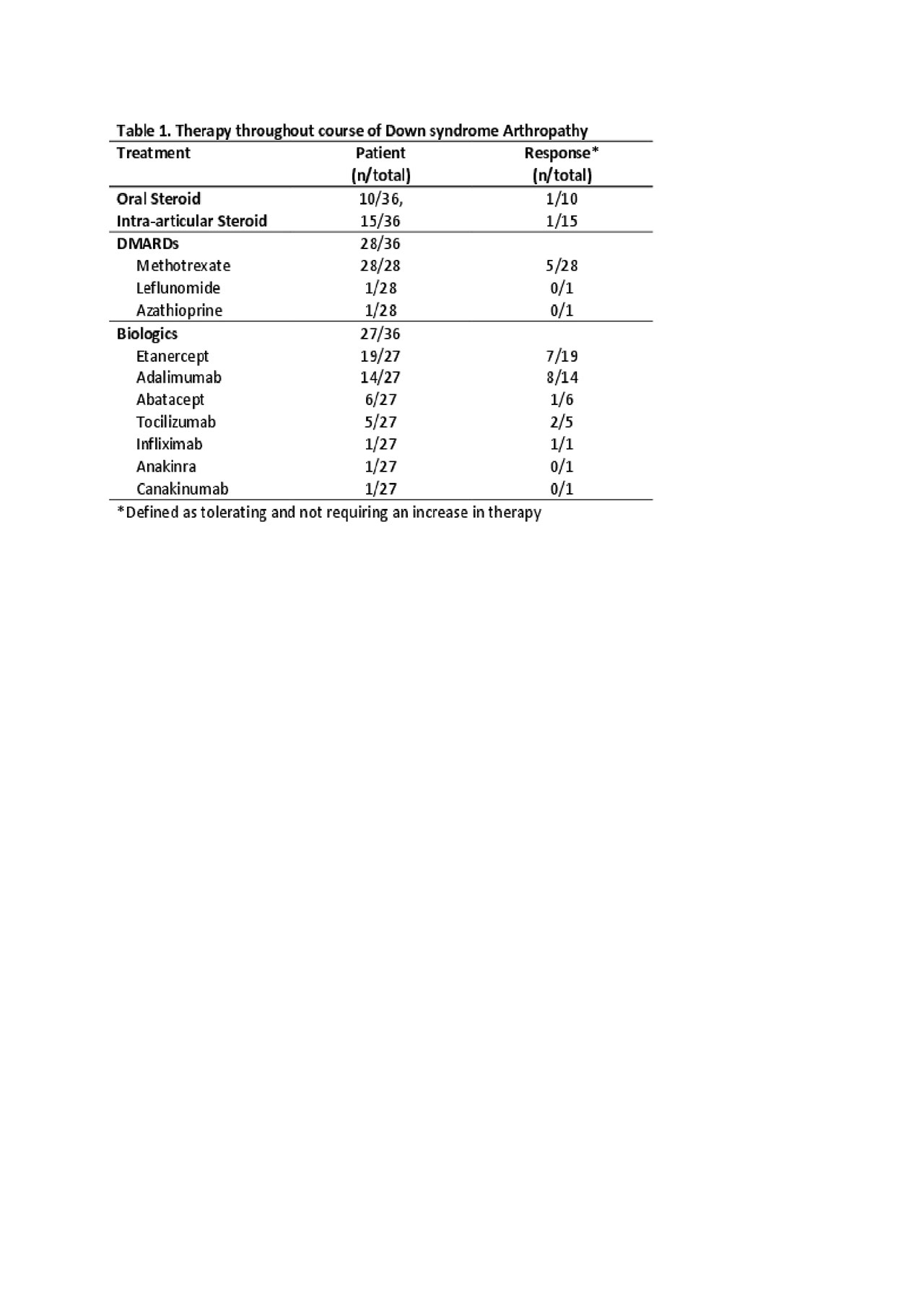
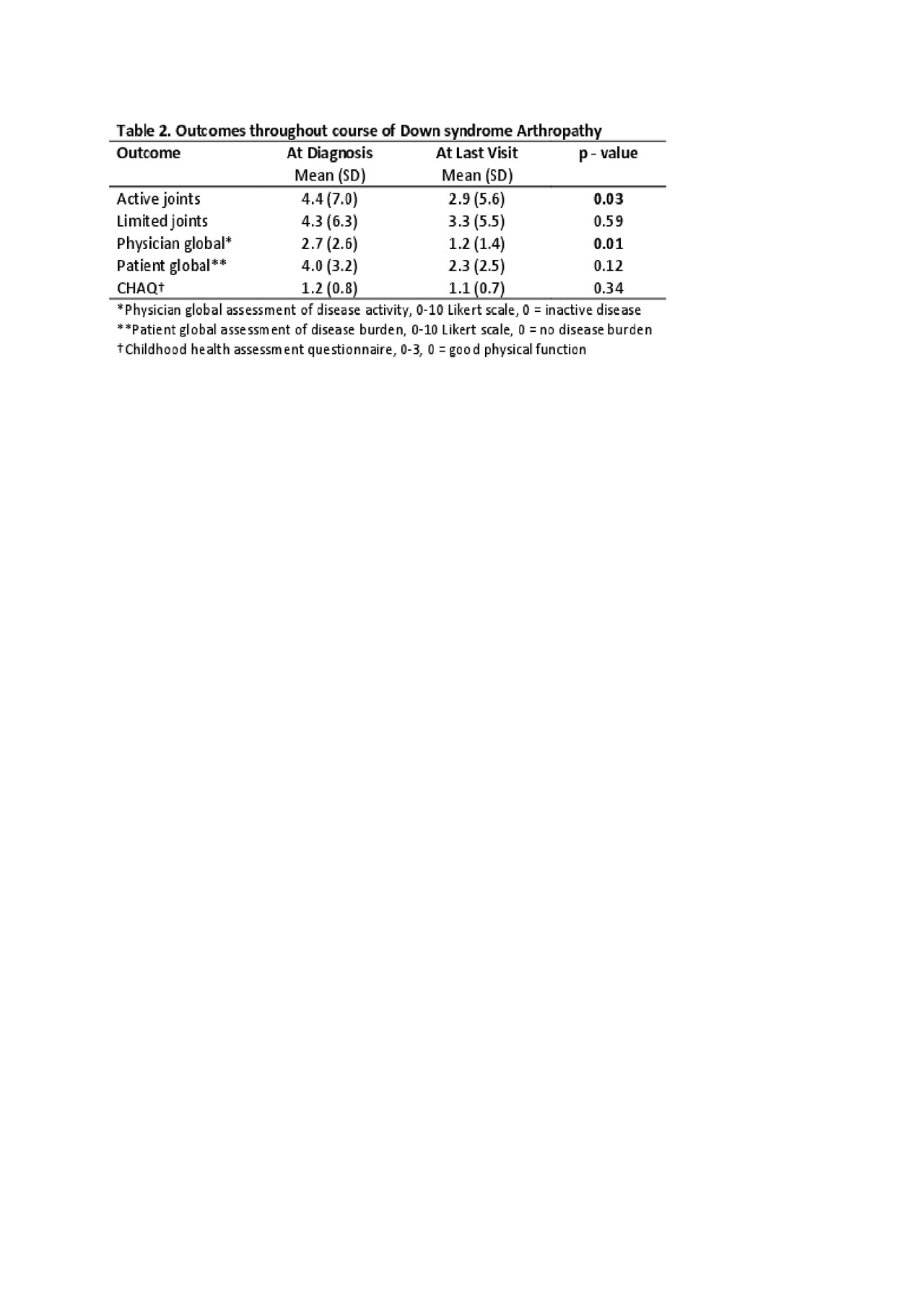
Results: Thirty-six patients with DA were identified in the total population of 7337 JIA patients (0.005%) in the Registry with a mean follow-up period of 4.5 years (SD 3.2). The mean age at onset of musculoskeletal symptoms was 7.1 years (SD 3.9) and the mean months to JIA diagnosis was 11.5 months (SD 21.6). At diagnosis, 64% had a polyarticular RF negative presentation, 39% reported morning stiffness with an average of 4 active (SD 7; range 0 – 27) and 4 limited joints (SD 6; range 0-23), and a mean physician global of disease activity of 2.7 (SD 2.6; range 0 – 9). At diagnosis, 22% had elevated inflammatory markers (CRP, ESR), while 33% and 17% were positive for ANA and HLA-B27, respectively. Over half the patients (64%) were started on a disease modifying antirheumatic drug (DMARD) at diagnosis with 36% simultaneously starting a biologic. Over the course of disease, 78% used a DMARD (100% used methotrexate [MTX] at some point) and 75% used a biologic (mostly etanercept [70%]). Of those exposed to DMARD therapy, fifteen patients (54%) had at least one change of their DMARD (most due to MTX adverse effect [46%]), however, at the last recorded visit, 42% were on methotrexate. Fifteen patients (55%) had at least one change in biologic therapy due to inadequate response to therapy (Table 1). Between the first and last visit significant (p < 0.05) improvements were seen for active joint count and physician global of disease activity (Table 2).
Conclusion: In a large multicenter observational registry of JIA patients in North America, patients with Down syndrome arthropathy continue to have an approximate year-long delay in diagnosis from symptom onset. Treatments included corticosteroids (oral and intra-articular), DMARDs and biologic therapies throughout the course of the disease. Although there was a statistically significant improvement in active joint count and physician global assessment, there was no significant difference in limited joints, CHAQ, or patient global assessment with treatment. Optimal therapy remains unclear and current barriers are DMARD intolerance and anti-TNF effectiveness. More research is needed to determine optimal therapeutic approach.
To cite this abstract in AMA style:
Jones J, Lovell D, Smith C, Becker M. The down Syndrome Arthropathy Cohort in the New Childhood Arthritis and Rheumatology Research Alliance (CARRA) Registry: Clinical Characteristics, Treatment and Outcomes [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/the-down-syndrome-arthropathy-cohort-in-the-new-childhood-arthritis-and-rheumatology-research-alliance-carra-registry-clinical-characteristics-treatment-and-outcomes/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/the-down-syndrome-arthropathy-cohort-in-the-new-childhood-arthritis-and-rheumatology-research-alliance-carra-registry-clinical-characteristics-treatment-and-outcomes/