Session Information
Session Type: Poster Session (Tuesday)
Session Time: 9:00AM-11:00AM
Background/Purpose: Nonspecific proteasome inhibitors, eg, bortezomib (BTZ), target both the constitutive and immuno- proteasome and are approved for the treatment of multiple myeloma. While BTZ has been used to treat refractory SLE and LN, it is associated with adverse events (AEs) that limit its use. KZR-616, a first-in-class selective immunoproteasome inhibitor, is highly active in murine SLE (Muchamuel et al. ARD 2018. 77:A685). In healthy volunteers (HV), KZR-616 subcutaneously (SC) at 30 and 45 mg weekly (QW) was shown to be safe, be well tolerated, and achieve target levels of immunoproteasome inhibition (Lickliter et al. ARD 2018. 77:A1413). We report here the preliminary safety and efficacy of KZR-616 in the Phase (Ph) 1b portion of the MISSION Study KZR‑616‑002 in active SLE patients (pts) (NCT03393013).
Methods: This open-label multicenter dose escalation trial enrolled SLE pts with SLEDAI ≥4 despite stable background immunosuppressants, antimalarials, and/or corticosteroids (≤20 mg prednisone equivalent). The pts received KZR-616 at 45 mg (Cohort 1), 60 mg (Cohort 2), or 30 mg stepped up to 60 mg (Cohort 2a) SC QW through Week 13 (W13) with 12 weeks of follow up. Enrollment in Cohort 2a continued until ≥6 pts had ≥4 weeks of dosing with 60 mg. Safety data included AEs, vitals, ECGs, and laboratory tests. Pharmacokinetics (PK) and pharmacodynamics (PD) were assessed at baseline (BL) and W5. Efficacy measures included the SLEDAI, Cutaneous Lupus Erythematosus Disease Area and Severity Index (CLASI), 28 tender (T) and swollen (S) joint counts (JC), Physician (Ph) and Patient (Pt) Global Assessments (GA), and Patient Pain (PtP) in evaluable pts (receive ≥1 month of KZR-616; non-evaluable pts were replaced). No corrections were made for missing data.
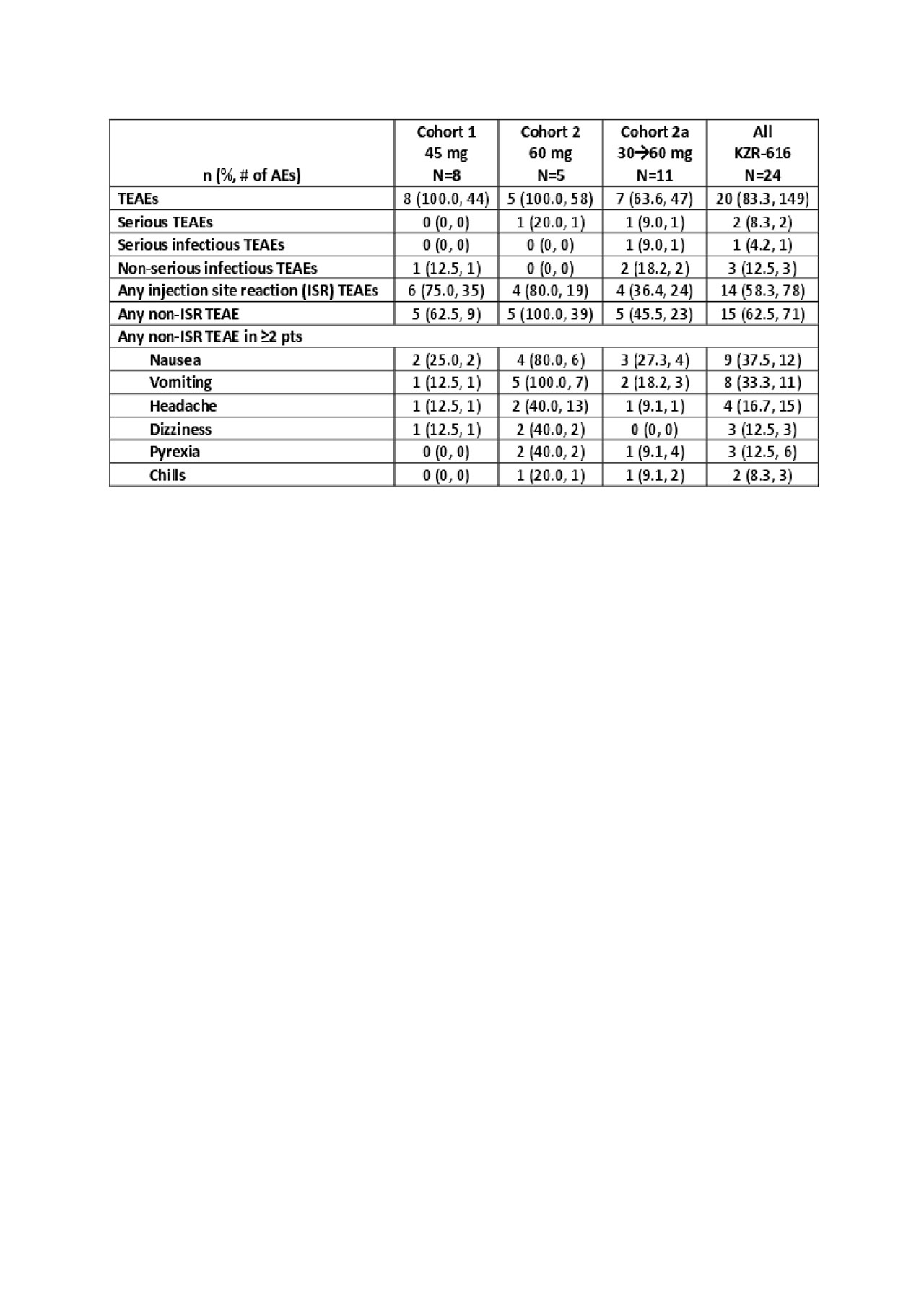
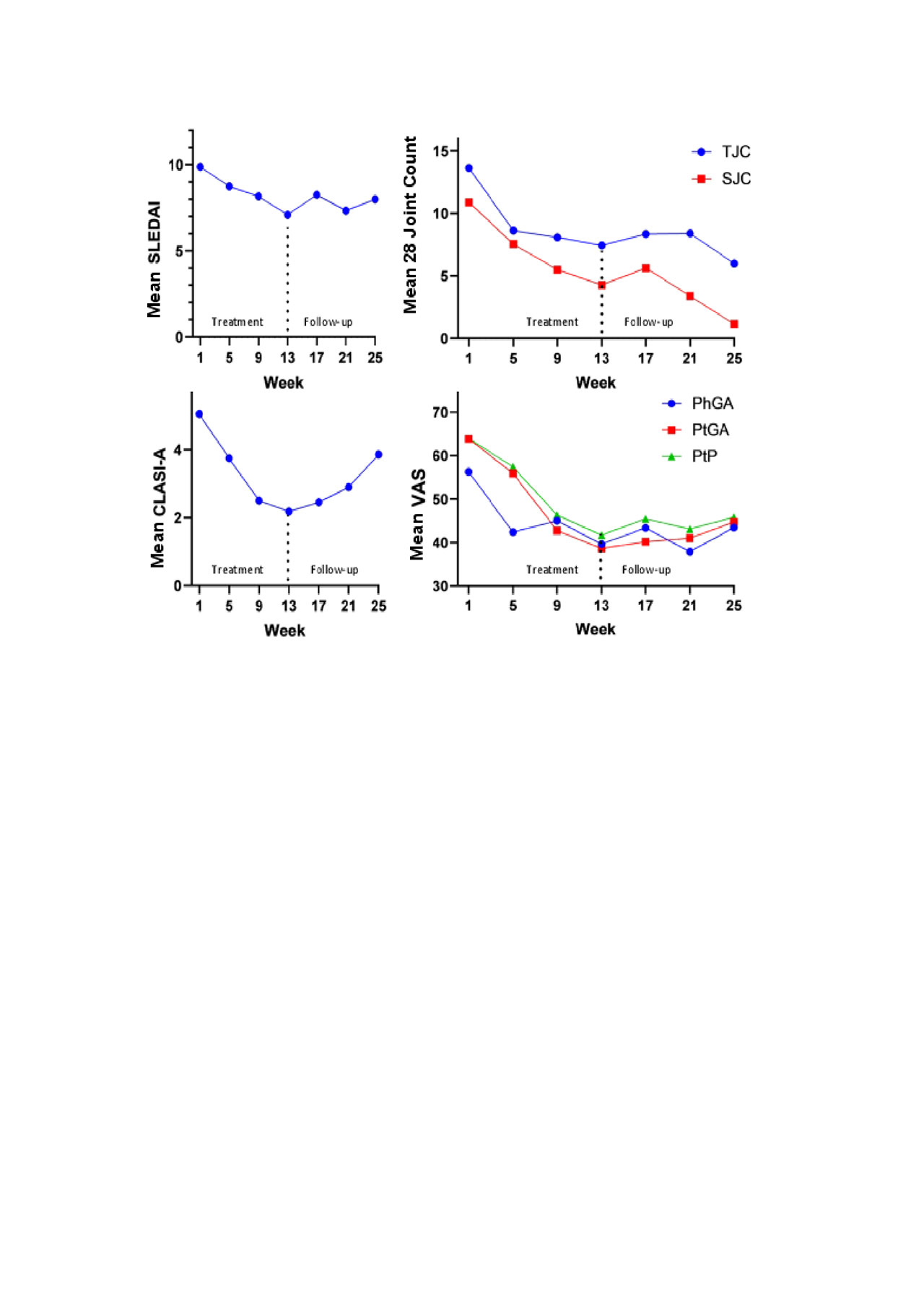
Results: Twenty-four pts were enrolled as of 6 May 2019 (Table 1): 91.7% were female; BL median SLEDAI was 10.0. All pts received ≥1 dose of KZR-616. In each cohort, 3 pts discontinued early: 3 due to withdrawn consent in Cohort 1; 1 due to AE and 2 due to withdrawn consent in Cohort 2; 1 due to loss to follow-up and 2 due to AEs in Cohort 2a. The majority of AEs were mild (85.8%) to moderate (12.8%) with no signs of peripheral neuropathy or clinically significant hematologic AEs. One pt in Cohort 2 had a serious AE (SAE) of thrombotic microangiopathy and withdrew from the study. In Cohort 2a, there was 1 SAE of localized herpes zoster; the pt resumed and completed dosing after SAE resolution. All pts in Cohort 2 vomited within ~8-24 hours (h) of their first dose (typically resolving within 24 h); 2 of the 5 pts completed the study at either 45 or 60 mg. Cohort 2a pts (30 to 60 mg step up) tolerated 60 mg without significant vomiting. The PK and PD at 45 and 60 mg were similar to that measured for the same doses in HV. Figure 1 shows mean evaluable pt efficacy data. Of 11 pts reaching W13 to date, 3 of 6 with low BL complement levels had resolution; 5 (45.5%) had a SLEDAI improvement of ≥4 points from BL.
Conclusion: KZR-616 at 45 mg SC QW or with step up dosing to 60 mg appears safe and well tolerated and showed evidence of improvement in disease activity at W13 in active SLE pts on stable background therapy. The Ph 2 doses in the first randomized placebo-controlled trial with KZR-616 in active LN pts on mycophenolate and prednisone are 30 and 45 mg.
To cite this abstract in AMA style:
Furie R, Bomba D, Dall'Era M, Khan A, Soneira M, Anderl J, Wang J, Kirk C, Goel N. Treatment of SLE Patients with the Immunoproteasome Inhibitor KZR-616: Results from the First 3 Cohorts of an Open-Label Phase 1b Dose Escalation Trial [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/treatment-of-sle-patients-with-the-immunoproteasome-inhibitor-kzr-616-results-from-the-first-3-cohorts-of-an-open-label-phase-1b-dose-escalation-trial/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/treatment-of-sle-patients-with-the-immunoproteasome-inhibitor-kzr-616-results-from-the-first-3-cohorts-of-an-open-label-phase-1b-dose-escalation-trial/