Session Information
Date: Monday, November 11, 2019
Title: 4M117: RA – Treatments III: Cardiovascular Disease & Readmissions (1890–1895)
Session Type: ACR Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: Patients with rheumatoid arthritis (RA) have an increased risk of venous thromboembolism (VTE, which includes pulmonary embolism [PE] and deep vein thrombosis [DVT]) compared with the general population. However, population-based data on the risk of VTE among patients with newly diagnosed RA and new users of disease-modifying anti-rheumatic drugs (DMARDs) are limited. The objectives of our study were: 1) to estimate the incidence rate of VTE, PE, DVT among new users of biologic DMARDs (bDMARDs) and conventional DMARDs (cDMARDs) in RA patients. 2) To compare risk of VTE, PE and DVT between new users of bDMARDs and cDMARDs.
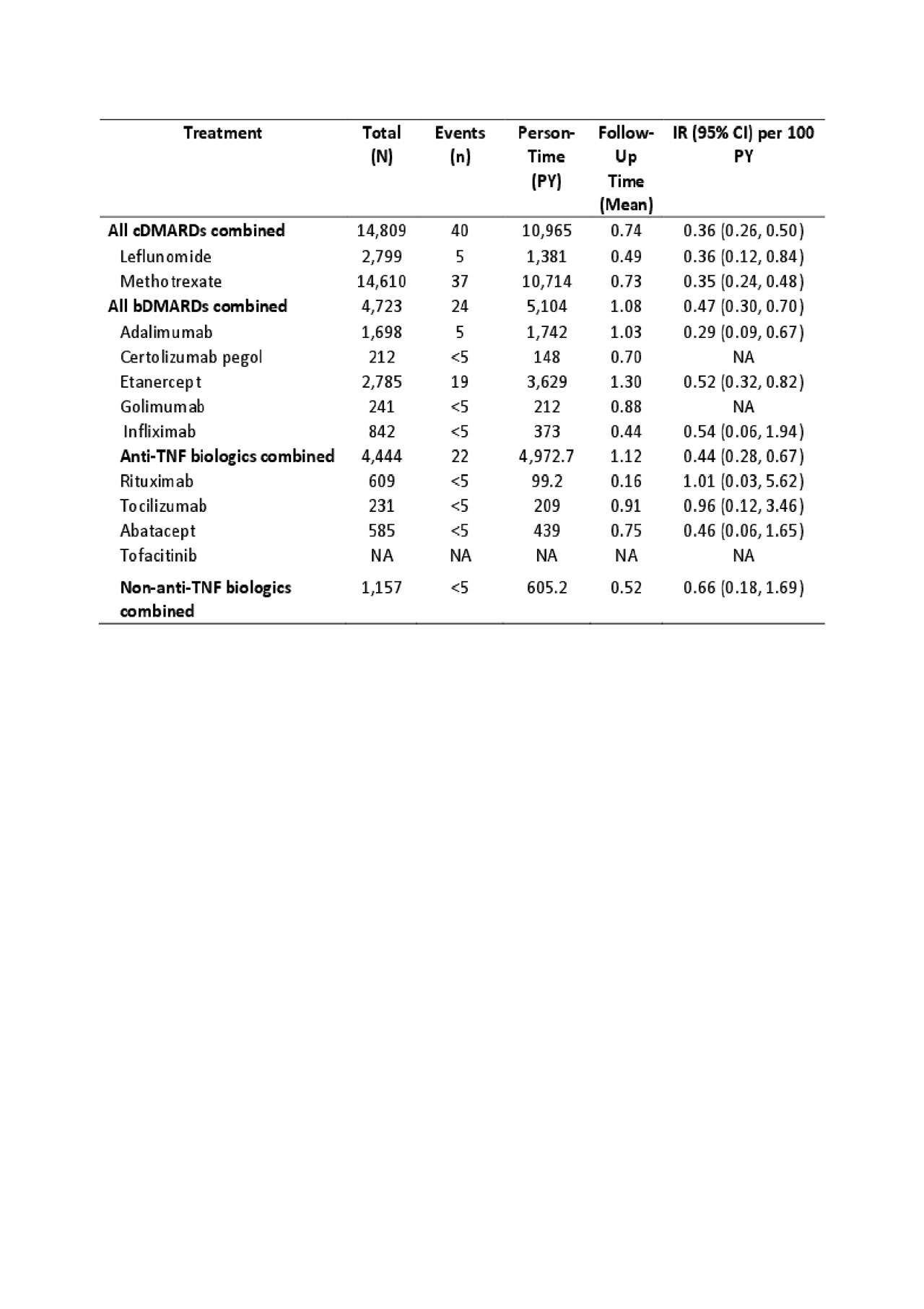
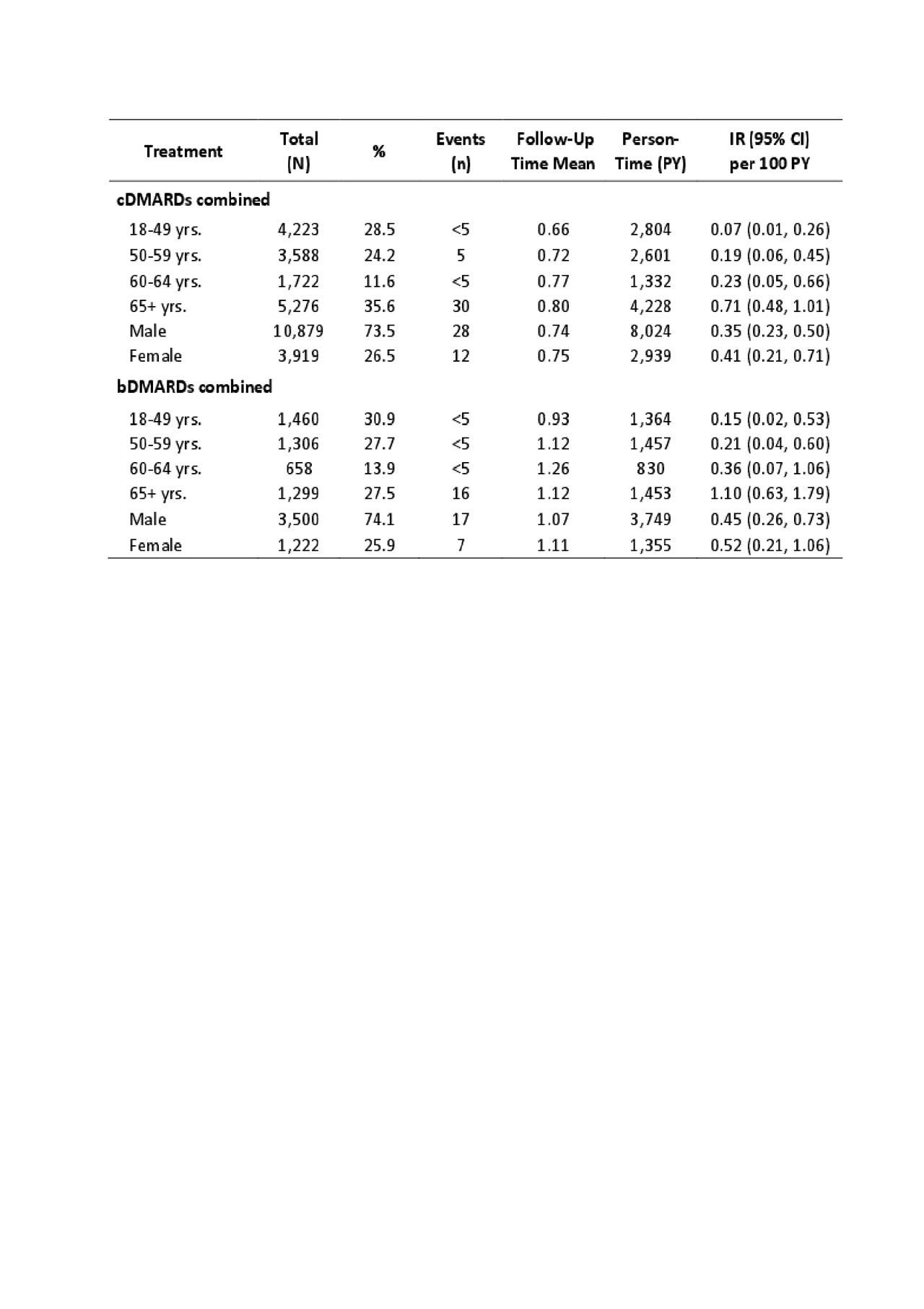
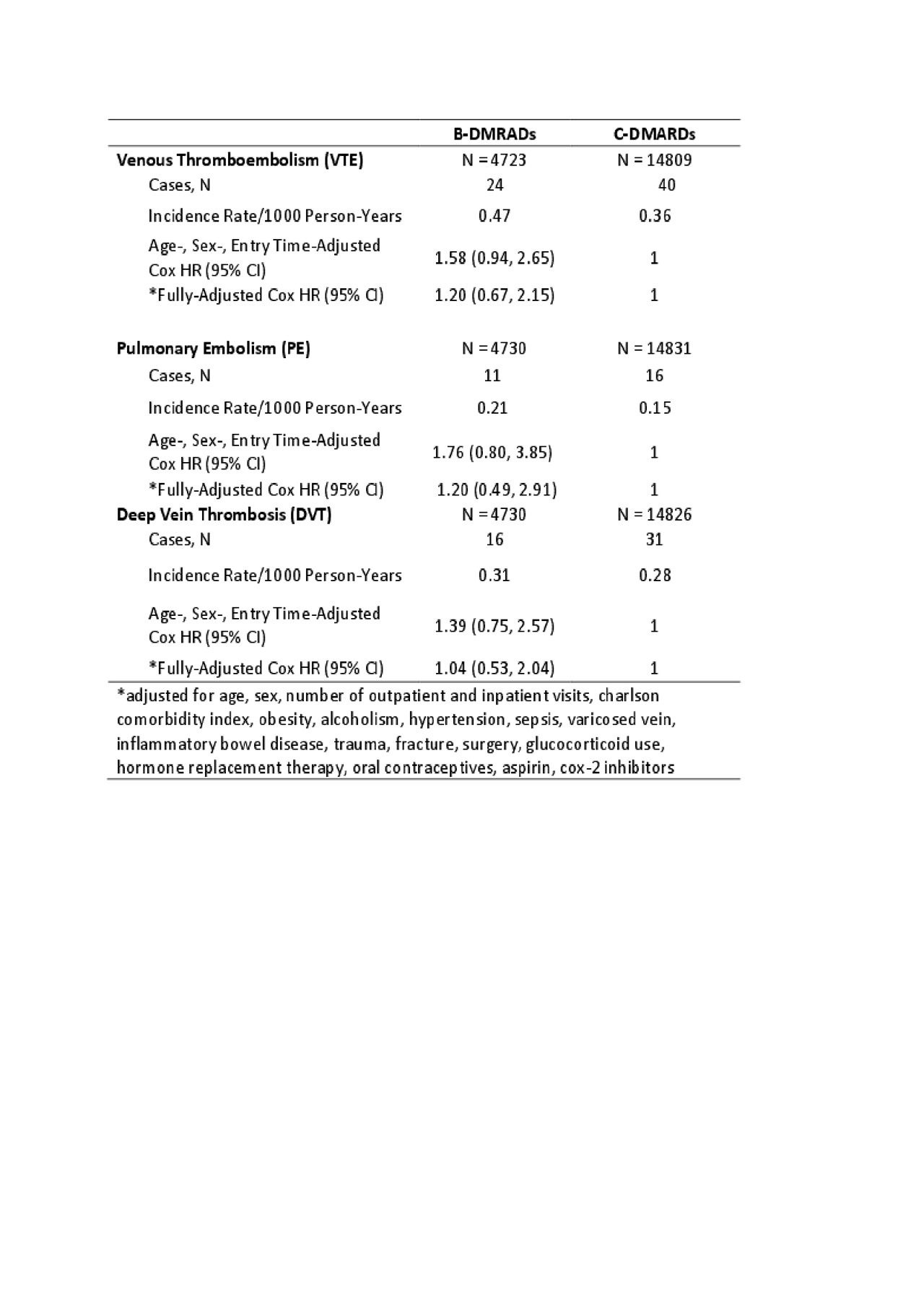
Methods: We conducted a population-based cohort study using administrative data that included all visits to physician and hospital from Jan 1990 until Mar 2015 and all dispensed medication from Sep 1995 to Mar 2015. Our sample included all patients with newly diagnosed RA using a previously validated case definition from physician billing data; and who were new users of a DMARD (no prior use in the 365 days before the first DMARD). Date of first DMARD use was considered the index date. For discontinuation, the exposure risk window for each DMARD extended until 30 days after the expiration of the last fill. Our outcomes of interest were first ever VTE, PE, and DVT based on ICD-9 or ICD-10 codes plus dispensation of an oral anti-coagulant. Follow-up began on the index date and continued until the earliest occurrence of: 1) DAMRD discontinuation; 2) leaving the province; 3) death; 4) the end of the study period; 5) the outcome of interest; or 6) switching to another DMARD. We calculated incidence rates per 100 person-years for each outcome (Table 1). Incidence rates of VTE were also stratified by age and sex. Furthermore, we conducted Cox proportional hazard regression models to estimate the hazard ratio (HR) of incident VTE, PE, and DVT during periods of bDMARDs use relative to periods of cDMARDs use after adjusting for baseline covariates.
Results: 14,809 patients were new users of cDMARDs (74% female, mean age of 58 yrs) and 4,723 were new users of bDMARDs (74% female, mean age of 56 yrs). Among new users of cDMARDs and bDMARDs, 40 and 24 patients developed VTE, the incidence rate was 0.36 and 0.47 per 100 person-years (Table 1), respectively. Across cDMARDs and bDMARDs, risk of VTE increased with age and was also higher in men than women (Table 2). Comparing bDMARDs with cDMARDs, the fully adjusted HRs were 1.20 (95% CI, 0.67-2.15), 1.20 (95% CI, 0.49-2.91), 1.04 (95% CI, 0.53-2.04) (Table 3), for VTE, PE, DVT, respectively.
Conclusion: The incidence rate of VTE varies by age and sex among RA patients treated with different DMARDs. The point estimate suggests a potentially increased risk of VTE in bDMARDs compared to cDAMRDs, but the results were not statistically significant. Further studies with larger sample size and adjusting for confounding by indication are needed.
To cite this abstract in AMA style:
Li L, Lu N, Lacaille D, Xie H, Esdaile J, Avina-Zubieta J. Risk of Venous Thromboembolism in Rheumatoid Arthritis Patients Initiating Biologic and Non-biologic DMARDs, a Population-based Study [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/risk-of-venous-thromboembolism-in-rheumatoid-arthritis-patients-initiating-biologic-and-non-biologic-dmards-a-population-based-study/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-venous-thromboembolism-in-rheumatoid-arthritis-patients-initiating-biologic-and-non-biologic-dmards-a-population-based-study/