Session Information
Session Type: Poster Session (Monday)
Session Time: 9:00AM-11:00AM
Background/Purpose: AS is a chronic, inflammatory disease of the axial skeleton associated with pain, stiffness, disability, and reduced quality of life (QOL).1 AS is defined by inflammation in spinal joints and radiographic changes at the sacroiliac joints, but patients (pts) frequently also have peripheral involvement, including swollen and tender joints (STJ),2 a feature associated with worse overall disease activity.3 Secukinumab is an IL-17 inhibitor approved for AS based on findings from the phase 3 MEASURE studies, in which it showed superiority to placebo in improving symptoms of AS.4-6 We explored whether peripheral STJ in pts with AS might be prognostic of outcomes following secukinumab treatment.
Methods: Data from MEASURE 1 (NCT01358175), 2 (NCT01649375), 3 (NCT02008916), and 4 (NCT02159053) were pooled from baseline to week 16 and included in this hypothesis-generating analysis. No adjustments for multiple comparisons were made. Pts with active AS received subcutaneous (SC) secukinumab every 4 weeks at doses of 300 mg with intravenous (IV) loading dose (LD), 150 mg with IV LD, 150 mg with SC LD, or placebo. Response to treatment was analyzed in pts with or without peripheral STJ at baseline. Peripheral joint involvement was assessed using a 44-joint STJ count. Response was assessed using multiple outcome measures, including Assessment in AS 20/40 (ASAS20/40), ASAS Partial Remission, BASDAI, BASFI, BASMI, disease activity and back pain assessments, and QOL questionnaires.
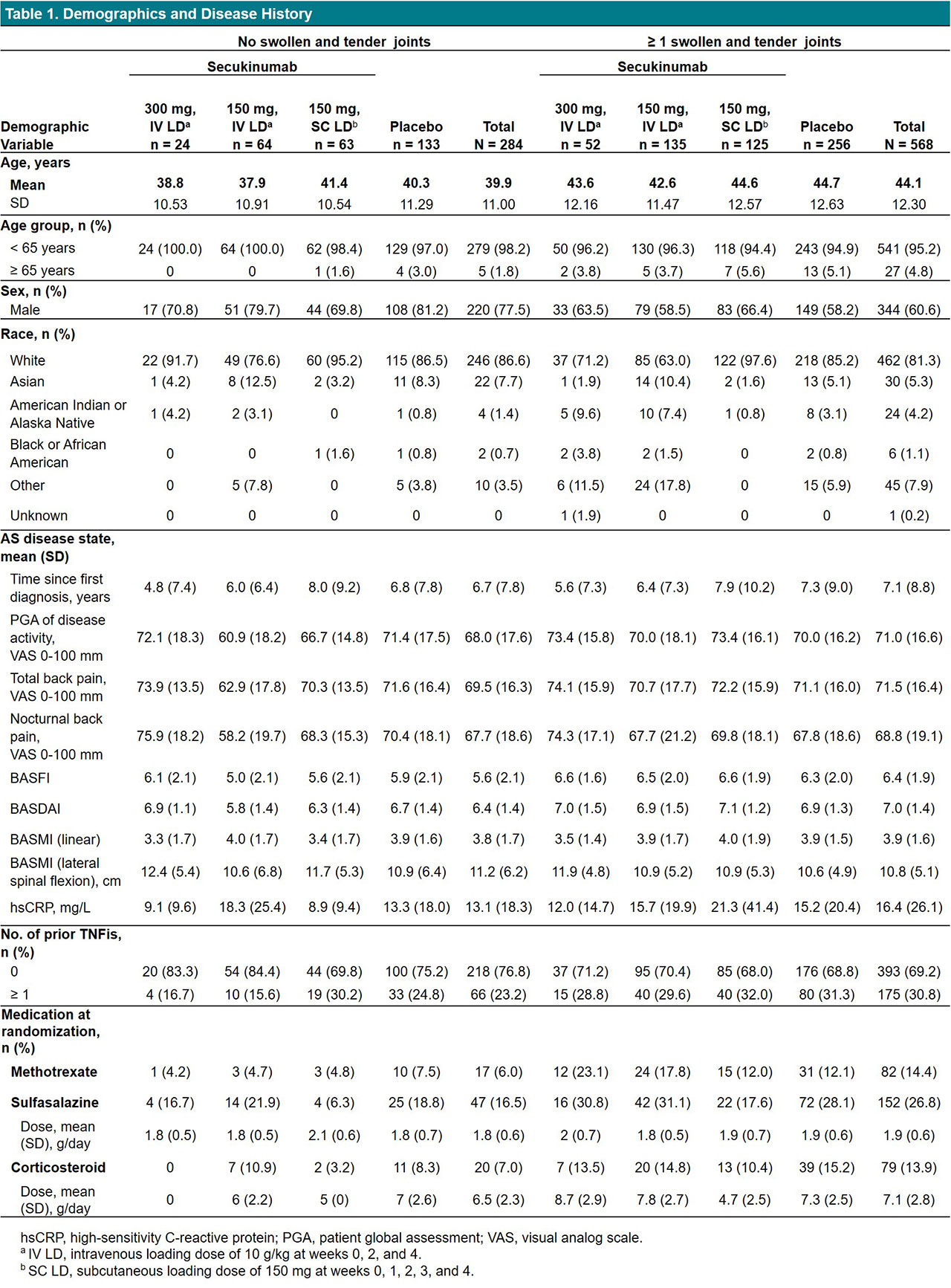
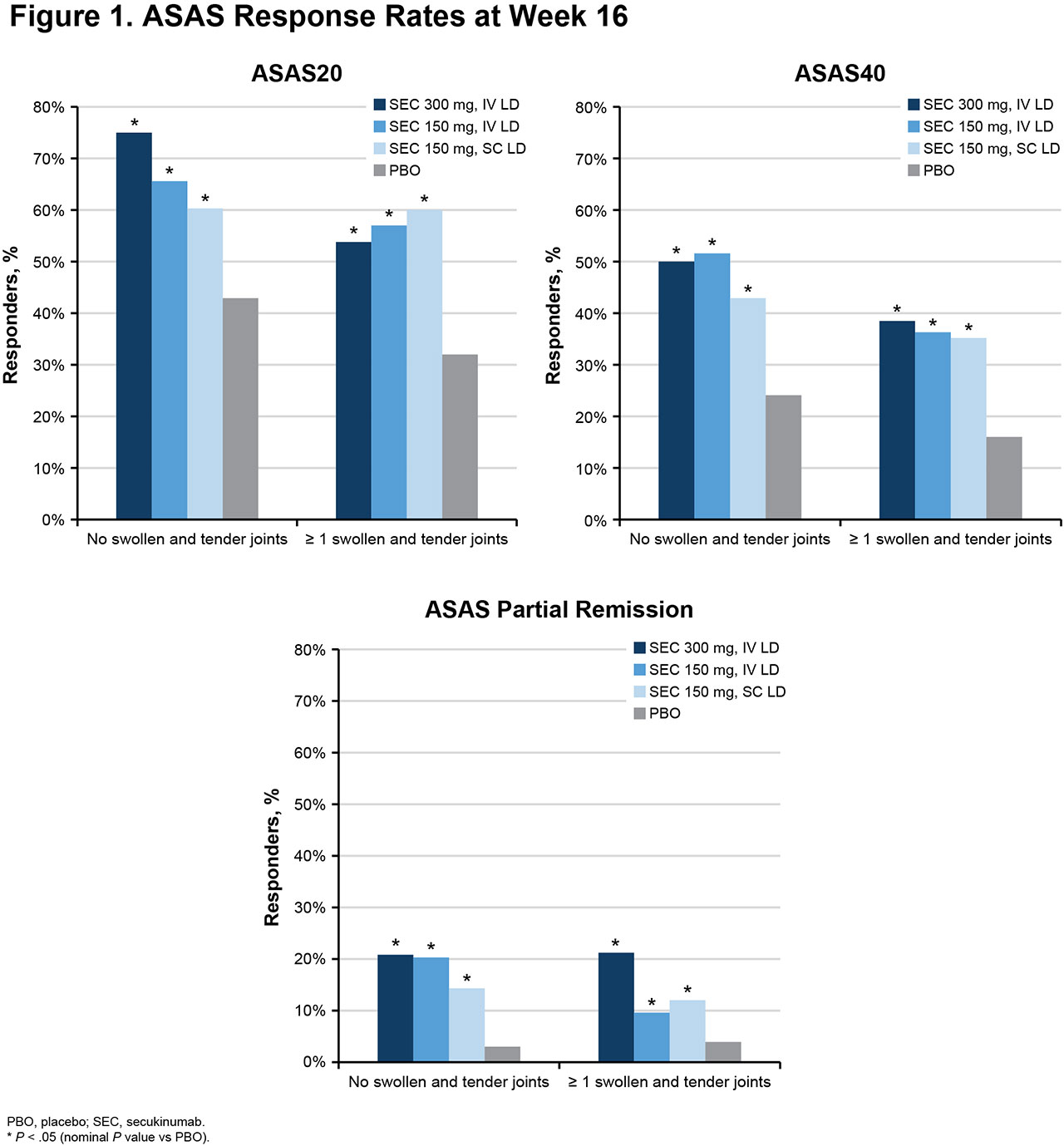
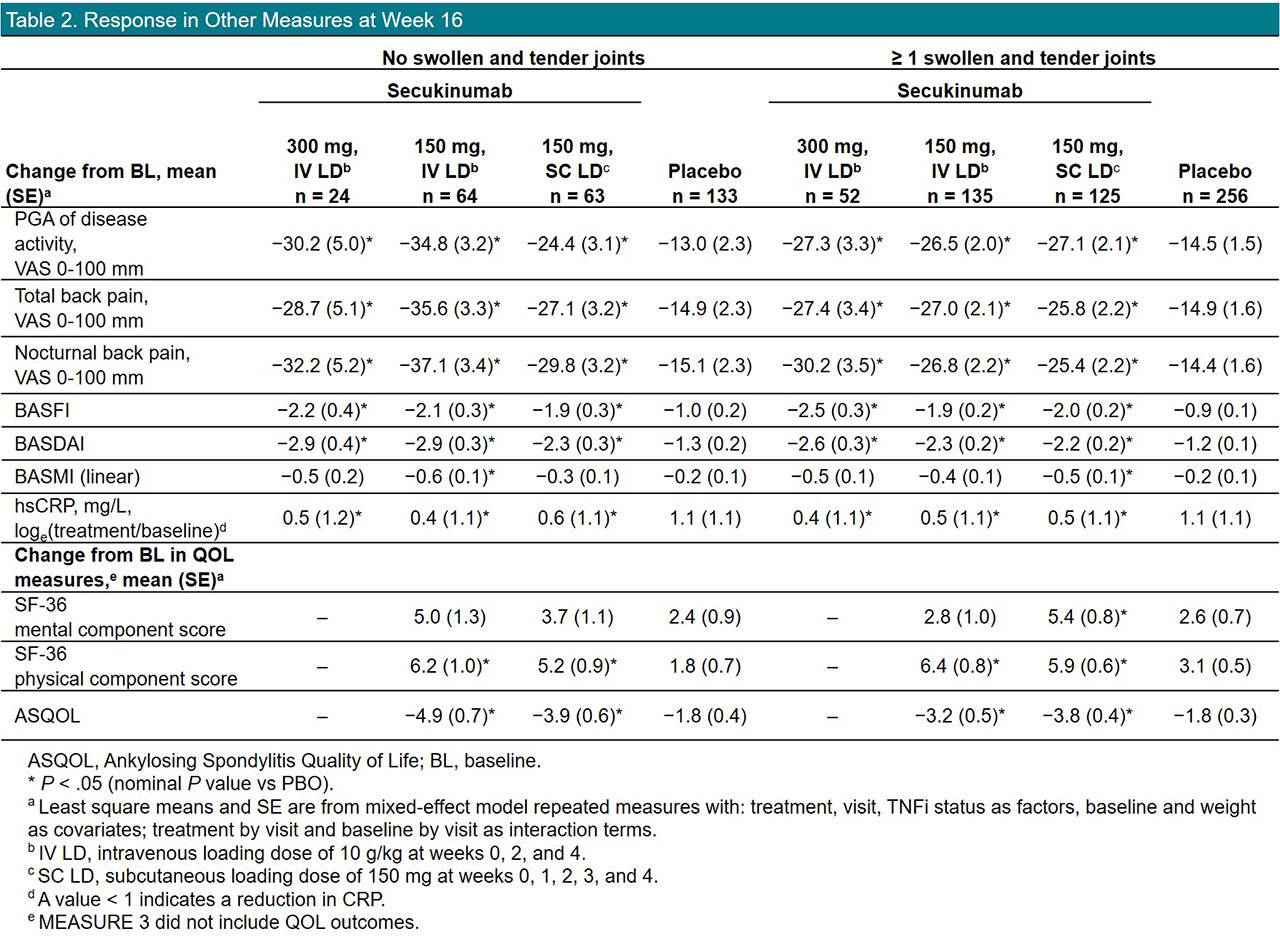
Results: Of 852 pts included in this pooled analysis, 814 completed 16 weeks of treatment with secukinumab (n = 450) or placebo (n = 364). On average, pts with no peripheral STJ were younger and more likely to be male; pts with STJ had higher CRP levels, had higher BASDAI and BASFI, had been diagnosed longer, and had more often received prior TNF inhibitors (TNFis) and DMARDs, suggesting more severe disease (Table 1). Overall, secukinumab led to significantly higher response rates than placebo in all outcome measures, regardless of the presence of STJ (Figure 1, Table 2). However, response rates tended to be numerically higher in pts with no STJ, with higher response rates seen for ASAS20/40 and ASAS partial remission (Figure 1). Overall, reductions in disease activity and back pain were also numerically greater in pts with no STJ (Table 2). QOL improvements were similar in pts with or without STJ.
Conclusion: Secukinumab led to significant improvements in efficacy outcomes, regardless of peripheral joint involvement. Pts with STJ were more difficult to treat, showing numerically lower responses to treatment.
References
1. Braun J, Sieper J. Lancet. 2007;369:1379-1390. 2. de Winter JJ, et al. Arthritis Res Ther. 2016;18:196. 3. de Winter JJ, et al. RMD Open. 2019;5:e000802. 4. Baeten D, et al. N Engl J Med. 2015;373:2534-2548. 5. Pavelka K, et al. Arthritis Res Ther. 2017;19:285. 6. Kivitz AJ, et al. Rheumatol Ther. 2018;5:447-462.
To cite this abstract in AMA style:
Mease P, Deodhar A, Calheiros R, Meng X, Fox T, Baraliakos X. Impact of Peripheral Swollen and Tender Joints at Baseline on Response to Treatment with Secukinumab in Ankylosing Spondylitis [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/impact-of-peripheral-swollen-and-tender-joints-at-baseline-on-response-to-treatment-with-secukinumab-in-ankylosing-spondylitis/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/impact-of-peripheral-swollen-and-tender-joints-at-baseline-on-response-to-treatment-with-secukinumab-in-ankylosing-spondylitis/