Session Information
Session Type: Abstract Submissions (ACR)
Background/Purpose: The aim of this study was to obtain cartilage-like constructs by chondrogenic differentiation of human bone marrow mesenchymal stem cells (hBM-MSCs) grown on different collagen scaffolds.
Methods: hBM-MSCs were characterized by flow cytometry using MSC markers and analysed for their multipotential differentiation capacity. These cells were cultured at a density of 200,000 cells/cm2 on different collagen scaffolds: Col I + Col II (C1C2); Col I + Col II + Heparan Sulfate (C1C2HS); Col I + Col II + Chondroitinsulfate (C1C2CHS); Col I + Heparin (C1OLH3). hBM-MSCs were cultured during 30 days in a chondrogenic differentiation medium supplemented with TGFβ-3. We have analysed cell growth and cell morphology by histochemical analysis and Transmission (TEM) and Scanning (SEM) Electron Microscope. Chondrogenic differentiation was confirmed by histochemical and immunohistochemical analysis. Moreover, we have tested relative gene expression of characteristic chondrocyte genes such us SOX9 and COLII. Finally, collagen concentration in the cultures supernatant was measured using a collagen Assay.
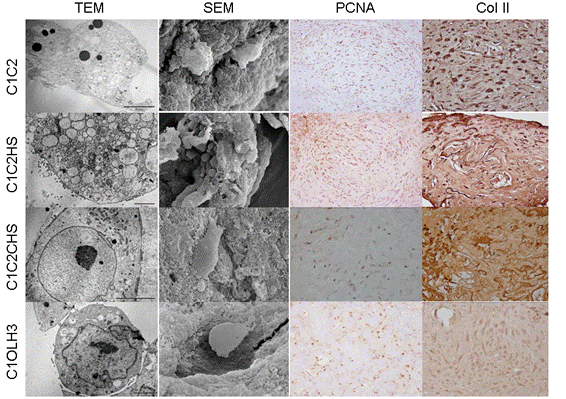
Results: hBM-MSCs were able to grow in the surface and inside of all the scaffolds and they were able to synthesize extracellular matrix (ECM) around the cells. Cellular proliferation was observed in all the scaffolds by PCNA (Proliferating Cell Nuclear Antigen) immunostaining, obtaining the highest values in C1C2 and in C1C2HS scaffolds. The percentage of cells, regarding the area of the analysed scaffold, was higher than 50% in C1C2 and C1OLH3 and higher than 75% in C1C2HS and C1C2CHS scaffolds. By means of immunostainings we detected Col II in the ECM of C1C2HS constructs and in the cytoplasm of the cells in C1C2 constructs. Collagen was detected in cultures supernatants of all the scaffolds obtaining the highest collagen release between 11-21 days of differentiation. These results indicated the beginning of the chondrogenic differentiation. TEM showed that cells had large number of lipid vesicles, mitochondrias and high secretory activity. SEM allowed us to study the adherence of cells, morphology and the ECM. We have detected the expression of two markers of early chondrogenesis, SOX9 and COLII, in all the constructs at 30 days.
Conclusion: Our data showed that the best results were obtained with C1C2HS constructs. hBM-MSCs cultured in chondrogenic medium over these scaffolds were able to differentiate better into chondrocyte-like cells and to synthesize more ECM than over the other scaffolds. Resulting cartilage‑like constructs may be suitable candidates for Tissue Engineering. Acknowledgements: Opocrin S.P.A.; CIBER-BBN CB0-01-0040; SAI-UDC.
Figure: Image obtained from the different constructs by electron microscopy analysis and immunohistochemistry. Vertically in columns going from top to bottom are shown the different types of scaffolds and ordered in rows from left to right are shown the different analysis.
Disclosure:
C. Sanjurjo-Rodriguez,
None;
A. H. Martinez-Sanchez,
None;
S. Diaz-Prado,
None;
E. Muiños-Lopez,
None;
I. M. Fuentes-Boquete,
None;
F. J. De Toro,
None;
F. J. Blanco,
None.
« Back to 2012 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/cartilage-tissue-engineering-using-collagen-scaffolds-and-human-mesenchymal-stem-cells/