Session Information
Session Type: Poster Session (Sunday)
Session Time: 9:00AM-11:00AM
Background/Purpose: The association between serum urate and hypertension continues to be a matter of controversy. Studies in adolescents provided evidence for the efficacy of urate lowering therapy to improve early hypertension, while at least one study in adults failed to find benefit. The Serum Uric acid Reduction to Prevent HypERtension (SURPHER) study tested whether serum urate reduction with allopurinol would lead to blood pressure reductions in pre-hypertensive young adults.
Methods: Single center, double-blinded, crossover trial in which participants were randomly assigned to allopurinol (300 daily mg) or placebo for a period of one month each separated by a 2-4 week washout. Adults ages 18-40 with baseline systolic blood pressure (SBP) ≥ 120 and < 160 mm Hg or diastolic blood pressure ≥ 80 and < 100 mm Hg, and serum urate ≥ 5.0 mg/dL for men or ≥ 4.0 mg/dL for women were enrolled. Main exclusion criteria included chronic kidney disease, gout, or use of urate-lowering therapies. The primary outcome was change from study treatment period baseline (allopurinol or placebo) in SBP assessed by 24 hour ambulatory blood pressure monitoring at four time points. Missing data was handled with a multiple imputation approach. Safety assessments were conducted as part of the study.
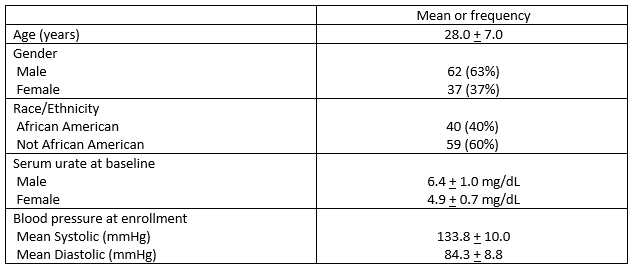
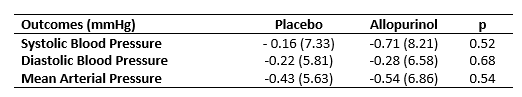
Results: 99 participants were randomized and 73 completed study participation. The characteristics of study participants are shown in Table 1. Serum urate decreased by 1.33 ± 1.21 mg/dL during the allopurinol period (p< 0.001) and by a non-significant 0.04 ± 0.75 mg/dL while taking placebo. SBP changed by -0.71 ± 8.21 mmHg during the period assigned to allopurinol versus -0.16 ± 7.33 mmHg during the period assigned to placebo. The difference between these changes in SBP was not significant (p=0.52) (Table 2). Changes in diastolic blood pressure and mean ambulatory blood pressure also were not significantly different during allopurinol and placebo exposure periods. There was a trend to significant blood pressure decreases in the small participant subset with average serum urate of >6.5 mg/dL at the initiation of study periods (Figure). No allopurinol hypersensitivity events or other serious adverse events were observed.
Conclusion: In the intention-to-treat analysis urate-lowering therapy with allopurinol in young adults did not lead to reductions in blood pressure when compared with placebo. Blood pressure reductions were observed in participants with high baseline serum urate levels.
To cite this abstract in AMA style:
Gaffo A, Calhoun D, Rahn E, Oparil S, Li P, Dudenbostel T, Redden D, Mudano A, Foster J, Feig D, Biggers S, Saag K. Effect of Serum Urate Lowering with Allopurinol on Blood Pressure in Young Adults [abstract]. Arthritis Rheumatol. 2019; 71 (suppl 10). https://acrabstracts.org/abstract/effect-of-serum-urate-lowering-with-allopurinol-on-blood-pressure-in-young-adults/. Accessed .« Back to 2019 ACR/ARP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/effect-of-serum-urate-lowering-with-allopurinol-on-blood-pressure-in-young-adults/