Session Information
Date: Sunday, October 21, 2018
Title: 3S102 ACR Abstract: Epidemiology & Pub Health I: Morbidity & Mortality (940–945)
Session Type: ACR Concurrent Abstract Session
Session Time: 4:30PM-6:00PM
Background/Purpose: While biologics are known to be associated with risk of serious infections, data on head-to-head comparison of different biologic drugs for the risk of infection are limited. We aimed to investigate the rate of incident serious bacterial, viral or opportunistic infection in rheumatoid arthritis (RA) patients starting tocilizumab (TCZ) versus TNF inhibitor (TNFi).
Methods: We conducted a cohort study using data from 3 U.S. healthcare claims databases: Medicare (2010-2015), ‘IMS’ PharMetrics Plus (2011-2015) and ‘Truven’ MarketScan (2011-2015). We identified patients with RA aged≥18 years who initiated TCZ or a TNFi with prior use of at least one different TNFi, abatacept or tofacitinib. Patients with recent infection, malignancy or rituximab use were excluded. The primary endpoint was incident composite serious infection including bacterial, viral or opportunistic infection with ≥1 inpatient principal diagnosis code. Secondary outcomes were specific subtypes of serious infection (Table). To control for >70 potential confounders including demographics, prior DMARD and antibiotic use, comorbidities, medications, and healthcare utilization in each database, TCZ initiators were propensity score (PS)-matched to TNFi initiators with a variable ratio of 1:3 within each database. We then calculated incidence rates (IR) and hazard ratios (HR) of the primary and secondary outcomes.
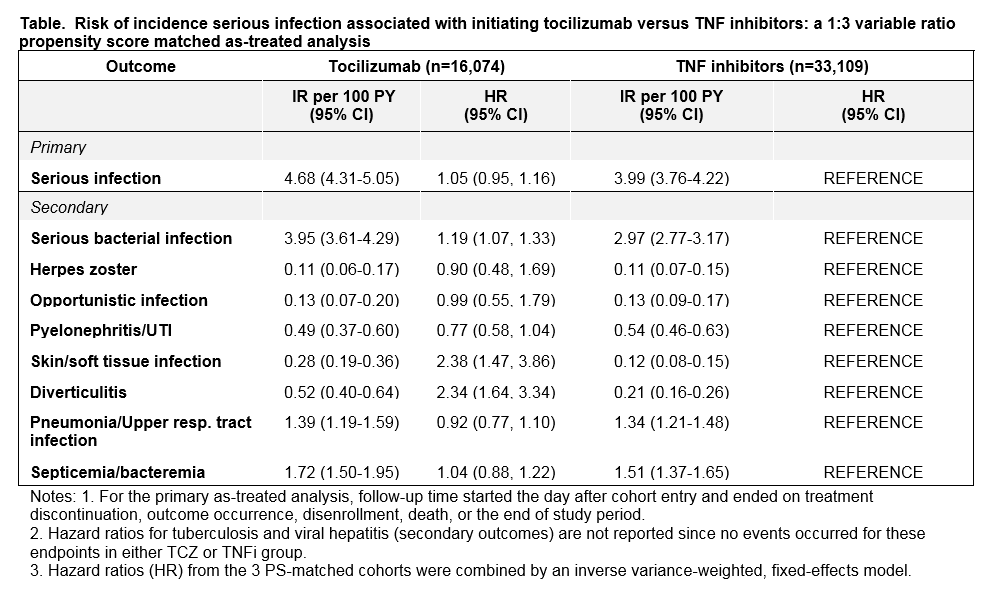
Results: A total of 16,074 TCZ initiators were PS-matched to 33,109 TNFi initiators. Mean age was 72 years in Medicare, 51 in IMS and 53 in Truven. At baseline, 69-73% patients used methotrexate and 70-79% used corticosteroids. In the as-treated analysis, the median follow-up time (days) ranged from 181 (MarketScan) to 213 (IMS) in the TCZ group and 198 (Medicare) to 235 (IMS) in the TNFi group. A total of 618 serious infections occurred in TCZ and 1,155 in TNFi group across the 3 databases. In the TCZ group, the IR for serious infections per 100 person-years ranged from 3.07 (Truven) to 7.05 (Medicare), and in the TNFi group, it ranged from 2.47 (Truven) to 7.05 (Medicare). The risk of incident composite serious infections was similar in TCZ versus TNFi initiators with a combined HR of 1.05 (95%CI 0.95-1.16) across all 3 databases. However, TCZ was associated with an increased risk of serious bacterial infection (HR 1.19, 95% CI 1.07-1.33), skin and soft tissue infections (HR 2.38, 95% CI 1.47-3.86) and diverticulitis (HR 2.34, 95% CI 1.64-3.34) compared to TNFi initiators. Secondary and subgroup analyses showed similar results.
Conclusion: This large multi-database cohort study found a similar risk for the composite primary endpoint of serious infection requiring hospitalization in RA patients who initiated TCZ versus TNFi after failing ≥1 biologic drug or tofacitinib. However, the risk of serious bacterial infection, skin and soft tissue infections, and diverticulitis was higher in TCZ initiators versus TNFi.
To cite this abstract in AMA style:
Pawar A, Desai RJ, Solomon D, Gale S, Bao M, Sarsour K, Schneeweiss S, Kim SC. Risk of Serious Infections in Tocilizumab Versus TNF Inhibitor Initiators in Patients with RA: A Multi-Database Cohort Study [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/risk-of-serious-infections-in-tocilizumab-versus-tnf-inhibitor-initiators-in-patients-with-ra-a-multi-database-cohort-study/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/risk-of-serious-infections-in-tocilizumab-versus-tnf-inhibitor-initiators-in-patients-with-ra-a-multi-database-cohort-study/