Session Information
Date: Sunday, October 21, 2018
Title: Rheumatoid Arthritis – Treatments Poster I: Strategy and Epidemiology
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Although biologics represent a major advance in rheumatoid arthritis (RA), many patients fail to achieve adequate responses to these agents. As previous studies have suggested that individual autoantibodies may selectively predict treatment response, we examined whether combined positivity to three well-characterized RA-related autoantibodies predicts treatment response among RA patients initiating biologics.
Methods: We examined data and banked serum from the Comparative Effectiveness Registry to study Therapies for Arthritis and Inflammatory Conditions (CERTAIN), a prospective RA cohort study. We examined a subset (n=1224) of enrolled patients including: biologic naïve anti-TNF initiations (35%), biologic exposed rituximab (10%) and tocilizumab (24%) initiations, and abatacept (31%) initiations regardless of previous biologic exposure. RF, anti-CCP and IgG antibodies to malondialdehyde-acetaldehyde (MAA) were measured centrally using banked enrollment serum. RF and anti-CCP positivity were defined per the manufacturer while anti-MAA positivity was defined as values >33rd percentile (to approximate the frequency of positivity for RF/anti-CCP). Response to therapy was evaluated at 6 months post biologic initiation. The relationship between the number of baseline autoantibodies (Abs) positive (range 0 to 3) with treatment response was examined using linear (DAS28 change) and logistic regression (>1.2 DAS28 change) accounting for age, sex, race/ethnicity, education, smoking, body mass index, concomitant DMARDs, baseline DAS28, and prior biologic use.
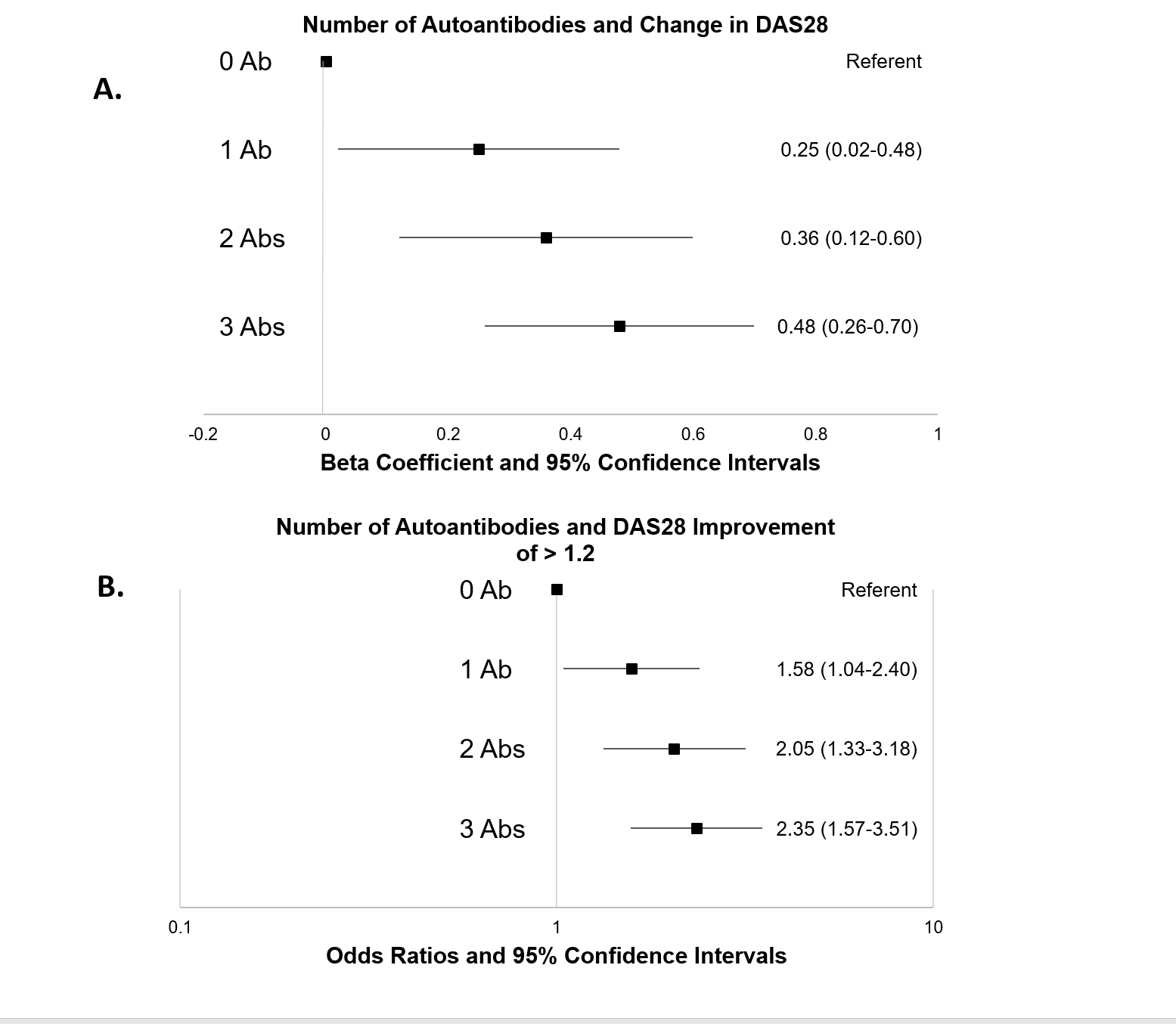
Results: Patients (n=1224) were predominantly Caucasian (89%) and female (79%) and had a mean age of 57 yrs. Most were RF (60%) or anti-CCP positive (55%) and biologic naïve (59%). Compared to those with no Abs positive, those positive for Abs achieved a significantly greater response (Figure). In separate univariate analyses, all 3 Abs were associated with DAS28 improvement with similar effects (β coef 0.29-0.31). After adjusting for covariates, patients positive for all 3 Abs had an average improvement in disease activity of 0.48 units (95% CI 0.26-0.70) compared to those with no Abs (A). Likewise, those with 3 positive Abs were 2.35 times more likely (95% CI 1.57-3.51) to achieve an improvement >1.2 DAS28 units during treatment (B). Associations between Ab positivity and treatment response were dose-dependent (trend p<0.001 in both models) and did not differ substantially across biologics.
Conclusion: An expanded Ab profile appears to significantly predict RA treatment response to biologic treatment in a dose-dependent fashion. Additional work that incorporates these serologic profiles with additional biomarkers or other informative patient characteristics could allow for the accurate prediction of treatment response, thus providing an opportunity to personalize RA management.
To cite this abstract in AMA style:
Mikuls TR, Bath A, England BR, Duryee MJ, Hunter CD, Kremer J, Pappas DA, Robinson WH, Curtis JR, Thiele GM. Expanded Autoantibody Profiles Predict Treatment Response to Biologic Therapy in Rheumatoid Arthritis [abstract]. Arthritis Rheumatol. 2018; 70 (suppl 9). https://acrabstracts.org/abstract/expanded-autoantibody-profiles-predict-treatment-response-to-biologic-therapy-in-rheumatoid-arthritis/. Accessed .« Back to 2018 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/expanded-autoantibody-profiles-predict-treatment-response-to-biologic-therapy-in-rheumatoid-arthritis/