Session Information
Date: Tuesday, November 7, 2017
Title: Osteoarthritis – Clinical Aspects Poster II: Observational and Epidemiological Studies
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
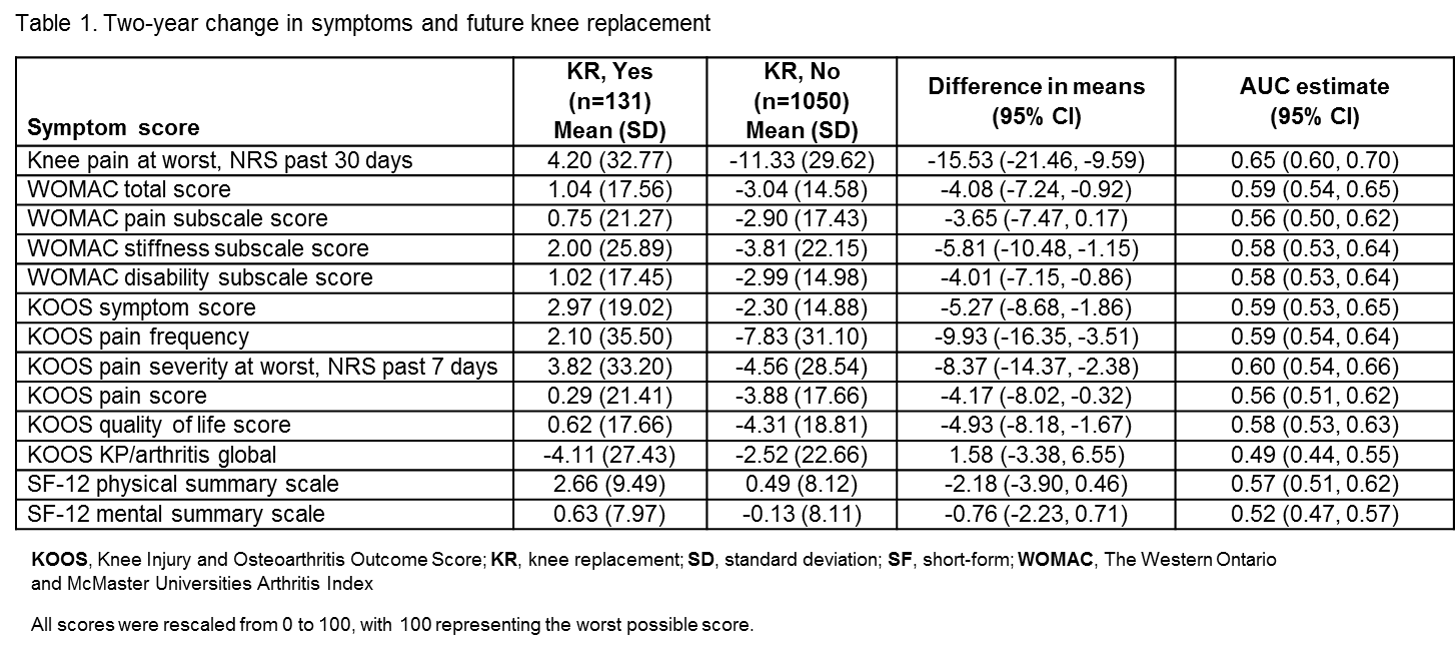
Background/Purpose: Randomized controlled trials (RCTs) of interventions for knee OA (KOA) may include a patient-reported outcome (PRO) measure as a primary endpoint. Several measures of KOA symptoms are potential candidates, although their comparative performance is unclear. The objectives of this study were to 1) estimate clinically important differences in 2-year changes in symptoms by anchoring change scores to a clinically important outcome, that is, future knee replacement (KR), and 2) compare 2-year changes in symptom scores with respect to their ability to discriminate knees that underwent KR over 7 years of follow-up.
Methods: OA Initiative knees were selected for analysis, based on eligibility criteria typical of a disease-modifying osteoarthritis drug (DMOAD) RCT for KOA, including Kellgren-Lawrence grade of 2 or 3, medial minimum joint space width ≥2.5 mm, knee pain at worst in the past 30 days from 4 to 9 on a 10-point numerical rating scale (NRS) or 0 to 3 if pain medication was taken for joint pain, excluding those with malalignment of >5º. KOA symptoms were assessed over 2 years, including knee pain severity over the past 30 days on a NRS, and over the past 7 days on a NRS, as well as WOMAC subscales, Knee injury and OA Outcome Score (KOOS) subscales, and the 12-item short-form health survey (SF-12). Scores were scaled 0–100, with 100 as the worst possible score. To characterize clinically relevant change, 2-year change in symptom scores was anchored to KR up to 7 years of follow-up and difference in mean change was estimated. To evaluate the ability of 2-year change in symptom scores to discriminate knees that went on to future KR, area under the receiver operating characteristic curve (AUC) was estimated and compared.
Results: The sample included 1,181 knees, with median follow-up of 5.8 years after the initial 2-year assessment, and 131 underwent KR. Two-year changes in WOMAC total, KOOS NRS 7 days, KOOS symptoms score, and NRS 30 days had similar discrimination for future KR (Table 1). When compared directly, the AUC for NRS 30 days was significantly better than other measures such as NRS 7 days (AUC difference = 0.05, CI = 0.01–0.10), total WOMAC (AUC difference = 0.06, CI = 0.01–0.11), and KOOS symptoms (AUC difference = 0.06, CI = 0.002–0.12).
Conclusion: Among knees that met typical eligibility criteria for a DMOAD RCT, 2-year change in WOMAC total, KOOS symptoms score, KOOS pain frequency, and KOOS pain severity NRS 7 days performed similarly, although the NRS 30 days was marginally better at discriminating future KR up to 7 years of follow-up. Investigators conducting RCTs of KOA interventions may consider each of these PRO measures as potential primary endpoints. Overall performance was modest, and additional work is needed to develop more responsive measures.
To cite this abstract in AMA style:
Kwoh CK, Guehring H, Ashbeck E, Hannon MJ, Aydemir A. Two-Year Changes in Knee Osteoarthritis Symptoms: Comparing Clinical Relevance of Patient-Reported Outcomes By Anchoring to Knee Replacement [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/two-year-changes-in-knee-osteoarthritis-symptoms-comparing-clinical-relevance-of-patient-reported-outcomes-by-anchoring-to-knee-replacement/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/two-year-changes-in-knee-osteoarthritis-symptoms-comparing-clinical-relevance-of-patient-reported-outcomes-by-anchoring-to-knee-replacement/