Session Information
Session Type: ACR Poster Session C
Session Time: 9:00AM-11:00AM
Background/Purpose:
Pegylated uricases are promising therapies for the treatment of severe chronic gout, particularly for the rapid resolution of tophi. However uricases are limited by the induction of anti-drug antibodies (ADAs) that can compromise efficacy and safety. We have developed SEL-212, a novel combination product consisting of pegsiticase co-administered with synthetic vaccine particles encapsulating rapamycin (SVP-R). Preclinical studies have shown the ability of SVP-R to induce durable, antigen-specific immune tolerance to a range of co-administered biologic drugs. A Phase 1 study of SEL-212 demonstrated mitigation of ADAs and sustained control of seum uric acid (sUA) for at least 30 days after a single dose. Here we report initial data on the safety, tolerability, and effects on sUA, ADAs, and gout fares of repeated monthly doses of SEL-212 from an ongoing Phase 2 clinical study in symptomatic gout patients.
Methods:
Patients with symptomatic gout (≥1 tophus, gout flare within 6 months, and/or gouty arthropathy) and elevated sUA (sUA ≥6mg/dL) were enrolled in SEL-212 treatment cohorts (N=6-10 patients/cohort) of fixed doses of pegsiticase (0.2mg/kg or 0.4mg/kg) alone or co-administered with SVP-R (0.05, 0.08, or 0.1mg/kg). SEL-212 was infused in 28-day cycles x3 doses followed by challenge with pegsiticase alone on 28-day cycles x2 doses. Safety, tolerability, sUA, and ADAs were monitored, and clinical data were collected.
Results:
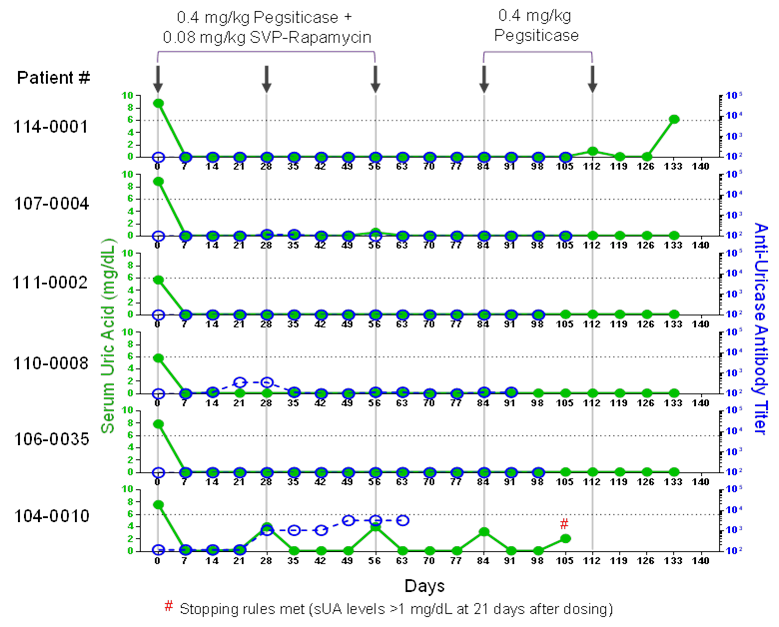
As of 12 June 2017, 60 patients had been dosed. A dose range matrix of pegsiticase and SVP-R enabled the identification of the minimum effective monthly dose (MED) as 0.4mg/kg pegsiticase+0.08mg/kg SVP-R. At the MED, 5/6 initial evaluable patients demonstrated sustained sUA levels <0.1 mg/dL with no or low ADA titers through 5 monthly doses, including two challenges with pegsiticase alone, and 4/6 demonstrated evidence of immune tolerance induction (Figure 1). In contrast, all patients treated with 0.4 mg/kg pegsiticase alone developed ADAs after the first dose and lost control of sUA by 14-21 days. Flare rates in the first 3 months were low for patients treated with SEL-212 (22%) versus those treated with pegsiticase alone (50%). SEL-212 was generally well tolerated at the clinically active doses. Of 7 observed serious infusion reactions, 4 were associated with patients receiving pegsiticase alone or the lowest dose of SEL-212, as expected, and two were related to dosing errors.
Conclusion:
SEL-212 has been well-tolerated, and, unlike pegylated uricases alone, has mitigated immunogenicity, reduced flare rate and enabled repeated monthly dosing with sustained control of sUA levels in gout patients.
Figure 1. SEL-212 control of sUA (green) and ADAs (blue)
To cite this abstract in AMA style:
Sands E, Kivitz AJ, DeHaan Ph.D. W, Johnston L, Kishimoto TK. Initial Phase 2 Clinical Data of SEL-212 in Symptomatic Gout Patients: Monthly Dosing of a Pegylated Uricase (Pegsiticase) with Svp-Rapamycin Enables Sustained Reduction of Serum Uric Acid Levels By Mitigating Formation of Anti-Drug Antibodies [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/initial-phase-2-clinical-data-of-sel-212-in-symptomatic-gout-patients-monthly-dosing-of-a-pegylated-uricase-pegsiticase-with-svp-rapamycin-enables-sustained-reduction-of-serum-uric-acid-levels-by-m/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/initial-phase-2-clinical-data-of-sel-212-in-symptomatic-gout-patients-monthly-dosing-of-a-pegylated-uricase-pegsiticase-with-svp-rapamycin-enables-sustained-reduction-of-serum-uric-acid-levels-by-m/