Session Information
Date: Monday, November 6, 2017
Title: Spondyloarthropathies and Psoriatic Arthritis – Clinical Aspects and Treatment Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Axial spondyloarthritis (axSpA), characterized by spinal inflammation, is also associated with extra-spinal manifestations including inflammation of the entheses (enthesitis). Enthesitis is equally prevalent throughout the axSpA disease spectrum and can severely reduce patients’ (pts) quality of life. RAPID-axSpA (NCT01087762) was a long-term, phase 3 study in axSpA pts with/without radiographic sacroiliitis treated with certolizumab pegol (CZP). Here we report improvements in tenderness at entheseal sites in males and females with axSpA treated with CZP for <4 years.
Methods: RAPID-axSpA was double-blind and placebo (PBO)-controlled to Wk24, dose-blind to Wk48 and open-label to Wk204. Pts fulfilled ASAS axSpA classification criteria with/without radiographic sacroiliitis (AS/nr-axSpA) and had active disease. Enthesitis was assessed by the Maastricht Ankylosing Spondylitis Enthesitis Score (MASES; count of 0–13 tender sites)1 at baseline (BL) and through to study completion/early withdrawal. Data are shown for all pts randomized to CZP (200 mg Q2W/400 mg Q4W) at BL, or where noted, for those reporting tenderness at BL. Observed case (OC) data are reported, with last observation carried forward (LOCF) imputation used where noted. Model-based analyses were run to evaluate effects independently associated with MASES outcomes (data not shown).
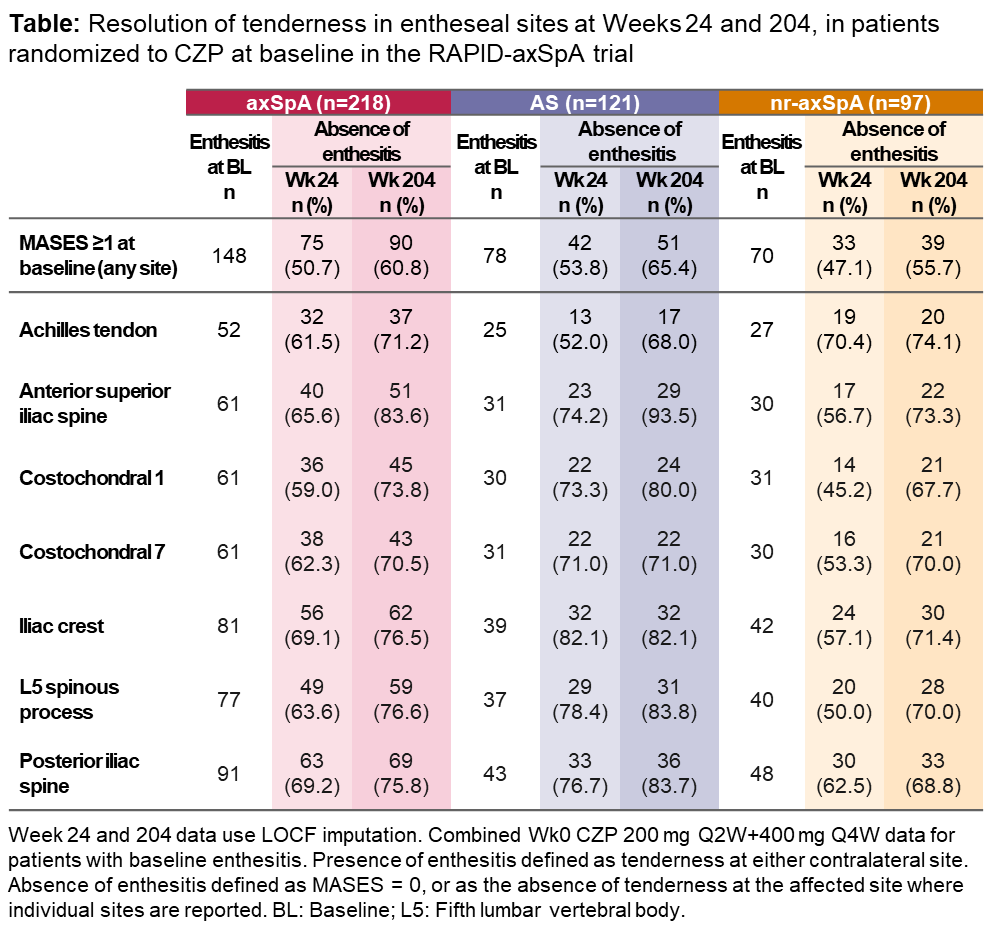
Results: Of 218 pts (83 female, 135 male) randomized to CZP at Wk0, 148 (67.9%) exhibited ≥1 tender site (MASES ≥1) at BL (AS: 78/121 [64.5%]; nr-axSpA: 70/97 [72.2%]). AxSpA females had mean (SD) MASES of 4.8 (4.0) at BL, with 84.3% reporting ≥1 tender site, compared to 57.8% for axSpA males whose mean (SD) was 2.7 (3.3). BL data were similar for AS and nr-axSpA females: 28/33 (84.8%) AS pts and 42/50 (84.0%) nr-axSpA pts reported ≥1 tender site and mean MASES = 4.6 and 4.9, respectively. At Wk204, mean MASES (OC/LOCF) was reduced to 1.0/1.1 (AS) and 2.0/2.5 (nr-axSpA). In males, mean MASES at BL = 2.4 (AS), 3.1 (nr-axSpA); Wk204 = 0.7/0.8 (AS), 0.6/0.7 (nr-axSpA). No notable differences were observed between entheseal sites in the resolution of tenderness at Wks24 and 204, with resolution in 70.5–83.6% sites affected at BL by Wk204 (Table). New onset of tenderness was rare: 1/107 pts (0.9%) without Achilles tenderness at BL reported tenderness at Wk204 (observed data).
Conclusion: In CZP-treated males and females across the broad axSpA spectrum with entheseal tenderness, improvements were maintained in all MASES sites. Females reported higher BL MASES than males. Improvements in MASES at Wk204 were greater for AS females than AS males, and were similar in both genders for nr-axSpA pts. A high percentage of pts with positive BL MASES at a particular site achieved total resolution in the affected area by Wk24, which was maintained to Wk204.
References:
1. Heuft-Dorenbosch L. et al. Ann Rheum Dis 2003;62(2):127–132.
To cite this abstract in AMA style:
Dougados M, Mease PJ, Sieper J, Taylor PC, de Peyrecave N, Nurminen T, Braun J. Improvements in Enthesitis Scores with Certolizumab Pegol Treatment in Males and Females with Active Axial Spondyloarthritis Are Maintained to Week 204 [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/improvements-in-enthesitis-scores-with-certolizumab-pegol-treatment-in-males-and-females-with-active-axial-spondyloarthritis-are-maintained-to-week-204/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/improvements-in-enthesitis-scores-with-certolizumab-pegol-treatment-in-males-and-females-with-active-axial-spondyloarthritis-are-maintained-to-week-204/