Session Information
Date: Monday, November 6, 2017
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: Until recently, most research on progranulin (PGRN) had been focused on its role in neurogenerative diseases as frontotemporal dementia. After the detection of elevated PGRN serum levels in patients with autoimmune arthritis we discovered antibodies against PGRN in a protein array-based screening of serum from various different rheumatic diseases. Furthermore, serum PGRN antibodies (PGRN-abs) have been proved to have neutralizing effect on PGRN serum levels; in addition, serum PGRN presented preferentially in a phosphorylated stage in PGRN-abs positive patients. Here we conducted the study to evaluate the prevalence of PGRN-abs in sera of rheumatoid arthritis patients being seronegative according to RF and ACCP.
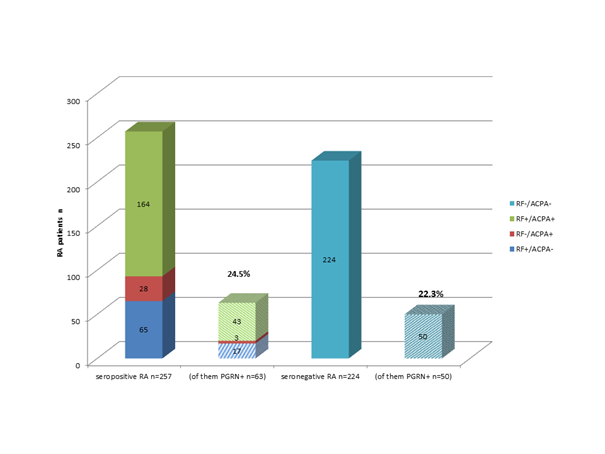
Methods: PGRN-abs were determined in sera of 257 RA patients with positivity for RF-IgM and/or ACCP-IgG and in sera of 224 seronegative RA patients, which were prospectively included in the study from 2013-2015. Healthy serum donors n=97 served as control cohort. All RA patients have fulfilled the revised ACR/EULAR classification criteria for RA from 2011. The ELISA detecting PGRN-abs as well as the phosphorylated stage of PGRN were applied as previously described. Subgroup analyses has stratified the RA cohort into diseases duration (less or more than 2 years) as well as gender, age of diagnoses, erosive course of diseases and the occurrence of tumor necrosis factor inhibitor failure (TNFi-failure). The statistics were performed with Mann-Whitney-U test evaluating the differences between seronegative, seropositive RA, and healthy controls; using the chi2 test differences between different RA subgroups were investigated. p-value of < 0.05 were considered significant.
Results: PGRN-abs were detected in 24.5% of seropositive RA and in 22.3% of RF/ACCP negative RA (OR 1.43, CI 0.93-2.21, p=0.106), Figure 1. In healthy controls (n=97) one serum was positive for PRGN-abs. Phosphorylated stage of PGRN were tested positive in sera of 33 of 36 PGRN-abs positive RA patients and negative in all 158 RA without PGRN-abs. Patients (n=107 out of 481) could be retrospectively stratified into early RA defined by diseases duration less than 2 years; early RA patients showed in 18.7% PGRN-abs compared to not-early RA patients (n=201; data missing) with 22.7% (OR 0.82; CI 0.52-1.30, p=0.397). Erosive disease as well as TNFi failure patients were significantly more frequently PGRN-abs positive (28.4%, 29.9%, respectively).
Conclusion: Here we present the first study evaluating the PGRN-abs in sera of patients with seropositive and seronegative RA. Testing PGRN-abs in sera of RA patients seems to reduce the fraction of seronegative status in RA patients by more than 20%. In what way PGRN-abs can be used as diagnostic biomarker has to be further demonstrated in larger cohort of RA patients.
To cite this abstract in AMA style:
Assmann G, Zinke S, Gerling M, Fadle N, Preuss KD, Pfreundschuh M, Thurner L. Serum Progranulin Antibodies (PGRN-abs) in Rheumatoid Arthritis Patients Being Negative for RF-IgM and ACCP-IgG [abstract]. Arthritis Rheumatol. 2017; 69 (suppl 10). https://acrabstracts.org/abstract/serum-progranulin-antibodies-pgrn-abs-in-rheumatoid-arthritis-patients-being-negative-for-rf-igm-and-accp-igg/. Accessed .« Back to 2017 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/serum-progranulin-antibodies-pgrn-abs-in-rheumatoid-arthritis-patients-being-negative-for-rf-igm-and-accp-igg/