Session Information
Date: Monday, November 14, 2016
Title: Rheumatoid Arthritis – Small Molecules, Biologics and Gene Therapy - Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: In patients with RA, obesity may impair clinical response to anti-TNF agents.1,2 In contrast, BMI does not appear to impact treatment retention or clinical response to abatacept (ABA);3 however, data on the effects of BMI are limited for patients with poor prognostic factors (radiographic erosion or RF/anti-citrullinated peptide antibody [ACPA] double seropositivity). Previously, poor prognostic factors were found to positively impact ABA retention, independently of treatment line.4 The current analysis tested the impact of BMI on ABA retention in biologic-naïve patients with RA who had poor prognostic factors at baseline.
Methods: ACTION is a 2-year, international, observational study of patients with RA who initiated IV ABA in routine clinical practice. Time to ABA discontinuation in biologic-naïve patients was estimated using Kaplan–Meier survival analysis. Clinically relevant variables, known risk factors, and potential predictive factors with significance in univariate models (p≤0.20) and no collinearity, were entered into multivariate Cox proportional hazards regression models. Prognostic factors of ABA retention were assessed in subgroups of patients with and without radiographic erosion at baseline. As comorbid obesity has previously been linked to a decreased likelihood of joint damage progression in patients with RA who are ACPA positive,2 BMI status was forced into the multivariate model after stratification for RF/ACPA double-seropositive versus double-seronegative status.
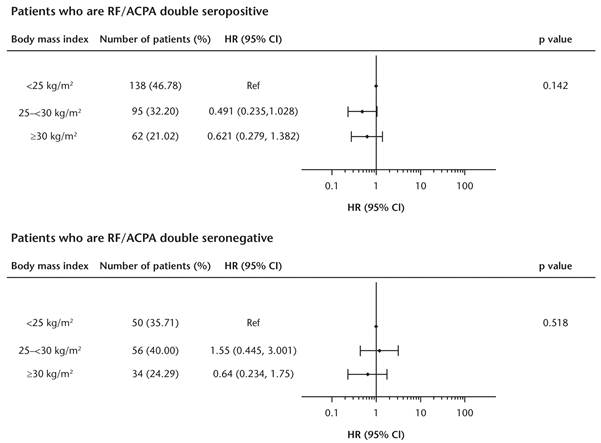
Results: Here we report 1-year results for 677 biologic-naïve patients with ≥1 prior conventional synthetic DMARD failure who enrolled into the ACTION study in two cohorts: 122 from Europe and Canada between May 2008 and December 2010; 555 from across Europe between September 2010 and December 2013. Data from the two enrollment cohorts were pooled owing to similar baseline characteristics. The 12-month ABA crude retention rate (95% CI) in biologic-naïve patients was 78.14% (74.72, 81.16). When forced into the multivariate model, BMI did not significantly impact ABA retention in the subgroups of patients who were RF/ACPA double seropositive (p=0.142) or double seronegative (p=0.518) at baseline (Figure).
Conclusion: Real-world evidence shows that RF/ACPA double seropositivity is predictive of abatacept retention in biologic-naïve patients.4 BMI does not significantly impact abatacept retention, even in subgroups of patients with poor prognostic factors such as RF/ACPA double seropositivity. 1. Gremese E, et al. Arthritis Care Res 2013;65:94–100. 2. Iannone F, et al. Joint Bone Spine 2015;82:187–91. 3. Nüßlein HG, et al. Clin Exp Rheum 2016;34:489–99. 4. Alten R, et al. Ann Rheum Dis 2016;75 (Suppl 2): 202.
To cite this abstract in AMA style:
Alten R, Lorenz HM, Nüßlein H, Mariette X, Galeazzi M, Cantagrel A, Chartier M, Elbez Y, Rauch C, Le Bars M. Body Mass Index Does Not Impact Abatacept Retention in Biologic-Naive Patients with Rheumatoid Arthritis Who Have Poor Prognostic Factors: A 12-Month Interim Analysis of an Observational, Prospective Study [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/body-mass-index-does-not-impact-abatacept-retention-in-biologic-naive-patients-with-rheumatoid-arthritis-who-have-poor-prognostic-factors-a-12-month-interim-analysis-of-an-observational-prospective/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/body-mass-index-does-not-impact-abatacept-retention-in-biologic-naive-patients-with-rheumatoid-arthritis-who-have-poor-prognostic-factors-a-12-month-interim-analysis-of-an-observational-prospective/