Session Information
Session Type: ACR Poster Session A
Session Time: 9:00AM-11:00AM
Background/Purpose: Polyglutamate metabolites of methotrexate (MTX) are believed to represent the pharmacologically active form of the drug and have been proposed as a biomarker to guide therapy in autoimmune arthritis. This work seeks to investigate the relationships between the formation of MTX polyglutamates and MTX efficacy in the collagen-induced arthritis (CIA) mouse model.
Methods: Arthritis was induced in male DBA/1 mice at 7-9 weeks of age by intradermal injection of chicken-collagen in complete Freund’s adjuvant (Day 0) with a booster injection at Day 19 (n=25) and compared to healthy controls (n=5). Subcutaneous MTX injections of 0, 2, 10, 20, and 50 mg/kg were given weekly beginning at Day 14 for a total of 6 weeks. Animal weight and arthritis disease activity were routinely assessed by paw volume measurements and a 16-point clinical disease score. Mice were sacrificed at Day 54 and tissue samples were collected. Joint and liver tissues were submitted for histopathologic scoring on a 16-point scale. Erythrocyte and liver samples were evaluated for MTX and its glutamate metabolites and plasma was analyzed for aspartate (AST) and alanine (ALT) aminotransferase activity.
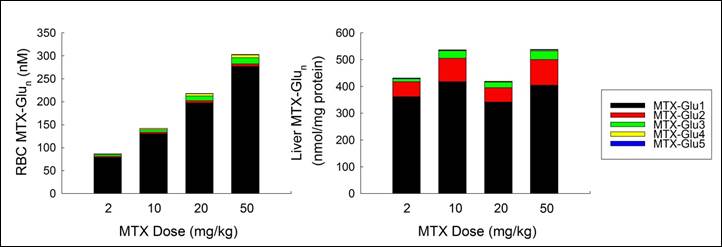
Results: The disease induction protocol resulted in 100% incidence of arthritis by Day 30 and an average ± SEM clinical disease score of 13.8 ± 1.3, a 69 ± 4% increase in paw volume, and a 10 ± 2 joint histology score in the positive control group by the end of the study. MTX treatment resulted in a dose-dependent reduction in disease activity. Maximum response was observed with the 20 mg/kg dose resulting in a reduced clinical disease score of 4 ± 4 (p<0.05), a reduction in paw volume expansion to only 12 ± 7% (p<0.001), and a reduced joint histology score of 1 ± 1 (p=0.02). Changes in animal weight were proportionate to disease activity, not MTX dose, and no hepatic toxicity was observed by Roenigk histological scoring or by AST/ALT evaluation. Erythrocyte concentrations of MTX were proportional to dose and ranged from 87 ± 2 nM at the 2 mg/kg dose to 303 ± 33 nM at the 50 mg/kg dose. In contrast, MTX concentrations in the liver were independent of dose and averaged 483 ± 21 nmol/mg of tissue. In contrast to human studies, significant accumulation of MTX polyglutamates was not observed in erythrocytes or liver tissue. Without regard to dose, the non-polyglutamated parent form of MTX accounted for 91 ± 0.4 % and 80 ± 1.5 % of total MTX in erythrocytes and liver tissue, respectively.
Conclusion: Once weekly subcutaneous MTX was effective in reducing the severity of collagen-induced arthritis in mice despite negligible tissue accumulation of MTX polyglutamates, and resulted in no measurable hepatotoxicity.
To cite this abstract in AMA style:
Singh R, van Haandel L, Kiptoo P, Becker ML, Siahaan T, Funk R. Evaluation of the Relationship Between Methotrexate Polyglutamation and Efficacy in the Collagen-Induced Arthritis Mouse Model [abstract]. Arthritis Rheumatol. 2016; 68 (suppl 10). https://acrabstracts.org/abstract/evaluation-of-the-relationship-between-methotrexate-polyglutamation-and-efficacy-in-the-collagen-induced-arthritis-mouse-model/. Accessed .« Back to 2016 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/evaluation-of-the-relationship-between-methotrexate-polyglutamation-and-efficacy-in-the-collagen-induced-arthritis-mouse-model/