Session Information
Session Type: ACR Plenary Session
Session Time: 11:00AM-12:30PM
Background/Purpose: Women with SLE and/or
aPL antibodies (SLE/APL) are at increased risk for adverse pregnancy outcomes (APO)
yet identification of those destined for complications remains challenging. Murine
studies implicate complement activation as an essential and causative factor in
fetal loss and growth restriction. Activation of the alternative complement
pathway, demonstrated by increased levels of the factor B fragment Bb, occurs
in non-autoimmune patients with preeclampsia. Activation of all complement
pathways leads to production of terminal complex C5b-9. We hypothesized that Bb
and soluble C5b-9 (sC5b-9) would be elevated in the circulation starting early
in the pregnancy of patients destined for APOs.
Methods: The PROMISSE Study (Predictors
of pRegnancy Outcome: BioMarkers In
antiphospholipid antibody Syndrome and Systemic Lupus Erythematosus)
enrolled pregnant women with ≥4 ACR SLE criteria and/or aPL antibodies
and healthy pregnant controls (HC). Subjects were evaluated and blood collected
monthly beginning at <12 wks gestation. Exclusion criteria were multi-fetal
pregnancy, prednisone >20mg/d, proteinuria >1gm/24hr, and creatinine
>1.2 mg/dL. APOs were: fetal death, neonatal death, preterm delivery <36
wks because of preeclampsia or placental insufficiency, and/or growth
restriction <5th %ile. Complement split products were measured on serial blood
samples in 492 SLE/APL patients and in 201 HC. Levels of Bb and sC5b-9 (by MicroVue™
Bb Plus and SC5b-9 Plus EIA Kits) were compared at each time point using analysis
of variance followed by pairwise comparisons with the Bonferroni method. Rates
of change in complement activation products through 27 wks of pregnancy were
estimated and compared using linear mixed effects models.
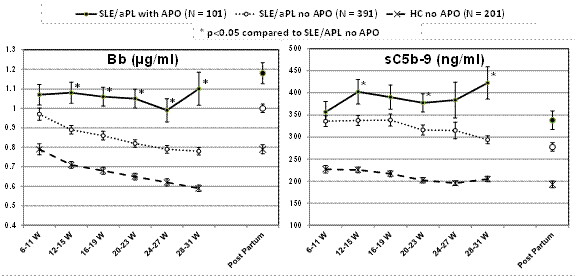
Results: APO occurred in 20.5%
of SLE/APL pregnancies. Compared to SLE/APL patients with no APO, Bb and sC5b-9
were significantly higher as early as 12-15 wks in those destined for pregnancy
complications and remained significantly elevated through 31 wks. Bb and sC5b-9
were significantly higher, starting as early as 12 wks, in SLE/APL patients
regardless of outcome, compared to HCs. In all three groups, there was a
decrease in Bb as pregnancy progressed, but the rate of decrease was
significantly smaller in patients with APO compared to SLE/APL without APO and
HC. Because complement activation often precedes and accompanies SLE flares,
it is of note that in patients without flares, increased Bb still predicted
poor outcomes.
To cite this abstract in AMA style:
Salmon JE, Kim M, Guerra M, Kaplowitz E, Laskin C, Petri M, Branch WD, Lockshin M, Sammaritano LR, Merrill JT, Stephenson MD, Khamashta M, Peaceman AM, Lynch A, Buyon JP. Complement Activation Predicts Adverse Pregnancy Outcome in Patients with SLE and/or aPL Antibodies [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/complement-activation-predicts-adverse-pregnancy-outcome-in-patients-with-sle-andor-apl-antibodies/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/complement-activation-predicts-adverse-pregnancy-outcome-in-patients-with-sle-andor-apl-antibodies/