Session Information
Date: Monday, November 9, 2015
Title: Systemic Sclerosis, Fibrosing Syndromes and Raynaud's - Clinical Aspects and Therapeutics Poster II
Session Type: ACR Poster Session B
Session Time: 9:00AM-11:00AM
Background/Purpose: SSc-Ca is a poorly understood vascular
complication of SSc that usually related to extreme constant pain and infection/amputation
risk for persons living with SSc-Ca (PLWSC). No recognized treatment exists for
SSc-Ca. There are no standardized measures of therapeutic responsiveness to assess
potential medication impact on SSc-Ca. Results here reflect the next phases of
PROM development.
Methods: Qualitative analysis from 31 PLWSC interviews revealed
insight into self-management, quality of life, mental/physical function and
natural history of SSc-Ca. Seventy-eight concepts were collectively reported
by participants. Of these, 21 items related to any sphere of function or body
structure were extracted by iterative analyses. Patient Research Partners
(PRP) experienced in SSc research and questionnaires, assisted in formulating
potential question stems. Nine PLWSC assisted in field-testing (FT) in face to
face interviews and by quantitative response to potential PROM items using a 0-5
Likert rating ‘usefulness’ of potential PROM item in indicating improvement/ worsening
of SSc-Ca – ‘not applicable to me’ was an option. Selection of preferred
vocabulary, time reference, context and response scale (RS) format, length,
verbiage and images were examined with all participants.
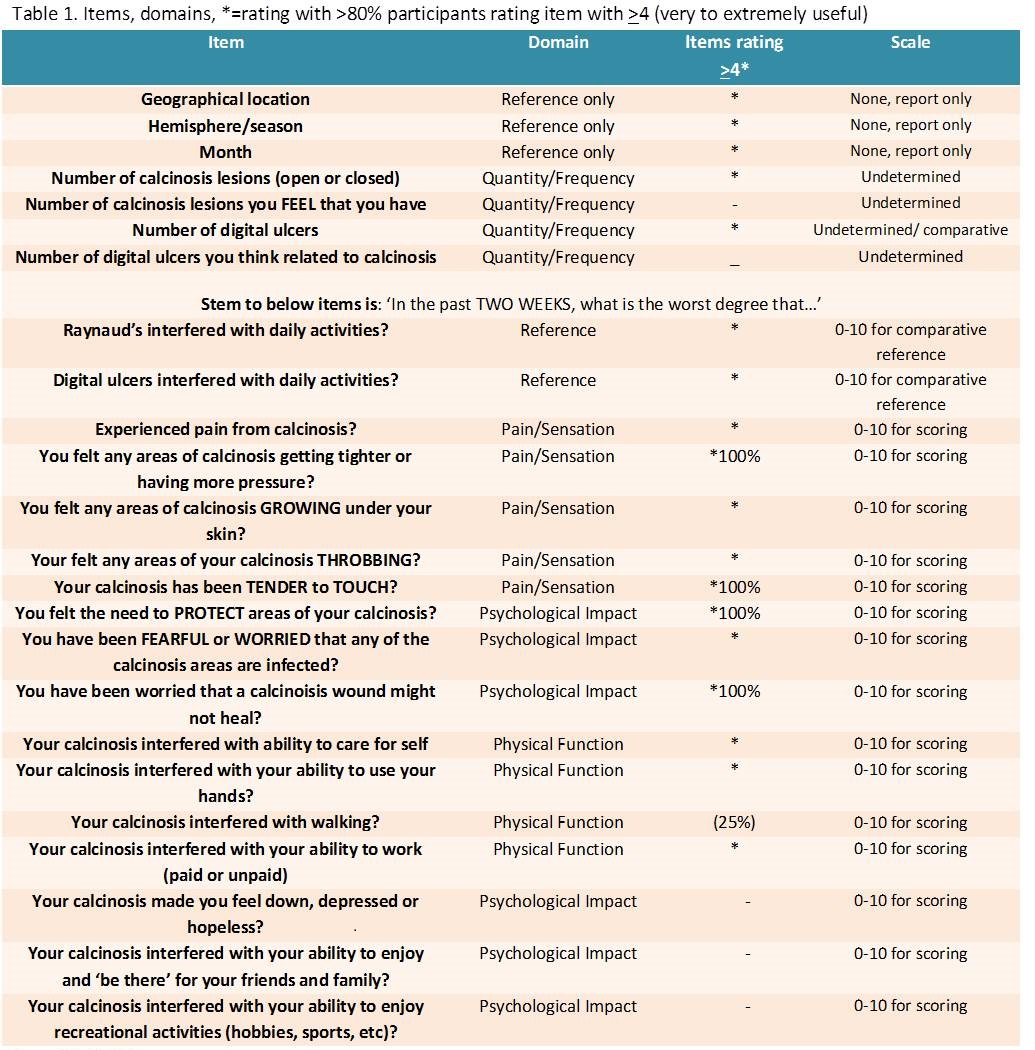
Results: Four PROM domains resulted: Quantity/Frequency (4
items), Pain/Sensation (5), Physical Function (4) and Psychological Impact (6).
The MCQ pre-test version is indexed in table 1. Two of 4 quantity items were
rated ≥4 by >80%, for which scoring are yet to be determined, quantile
scales are considered. For scalable items, there was 100% selection of a 0-10
scale over various formats 0-5, 0-7, 0-9 scales, >90% selected Likert
response over VAS, a reflective time reference of ‘2 weeks’, and a reflective
severity reference of ‘what is the worst degree’ with 100% selection for single
or brief word descriptors to cap scales i.e. ‘none’ to ‘worst possible’. Thirteen
of the 17 scaled items were rated >4 by >80% of participants and
100% 4 items. Two scaled questions pertaining strictly to Raynauds and digital
ulcers may be incorporated as ‘stabilisation’ questions scored separately for comparative
reference. Only the 2 participants experiencing foot calcinosis rated the ‘walking’
item highly; causing consideration for a test question combining upper and extremity
physical function in order to create a uniformly weighted instrument.
Conclusion: This is the first known SSc-Ca PROM developed
according to FDA Guidance. These items will undergo test/re-test validation
with subsequent multi-centre prospective assessment of the surviving MCQ items
beginning in July 2015. The MCQ is named in honor of Anne*Mawdsley*- an
original research team member, founder of the Raynauds & Scleroderma Association
UK, and a PLWSC who raised over ₤10 million for SSc research, education
and advocacy in her lifetime.
To cite this abstract in AMA style:
Saketkoo LA, Fligelstone K, Busman E, Christensen A, Cenac S, Khalique S, Aubin A, Jaeger VK, Mawdsley A, Gordon JK, Kaufman R, Baron M, Steen VD, Frech TM. Development of the Mawdsley Calcinosis Questionnaire (MCQ) Version 1 – a Patient-Reported Outcome Measure (PROM) for Systemic Sclerosis Related Calcinosis (SSc-Ca) [abstract]. Arthritis Rheumatol. 2015; 67 (suppl 10). https://acrabstracts.org/abstract/development-of-the-mawdsley-calcinosis-questionnaire-mcq-version-1-a-patient-reported-outcome-measure-prom-for-systemic-sclerosis-related-calcinosis-ssc-ca/. Accessed .« Back to 2015 ACR/ARHP Annual Meeting
ACR Meeting Abstracts - https://acrabstracts.org/abstract/development-of-the-mawdsley-calcinosis-questionnaire-mcq-version-1-a-patient-reported-outcome-measure-prom-for-systemic-sclerosis-related-calcinosis-ssc-ca/